Human ACE2 overexpression stable HEK293T cell lines
 Validation Data Post Download
Cat No.: GM-SC-293T‐hACE2-01
Validation Data Post Download
Cat No.: GM-SC-293T‐hACE2-01
Order information
| Catalog No. | Package | Price(In USD) | Qty (Quantity) | Sum(In USD) |
|---|---|---|---|---|
| GM-SC-293T‐hACE2-01 | Vial | 4288 | ||
| Shipping Cost: | 960.00 | |||
| Total: | ||||
For GM-2019nCoV-PSV01 or PSV service, click here for inquiry. >>
Validation of hACE2 overexpression stable HEK293T cell lines (Cat. GM-SC-293T‐hACE2-01)
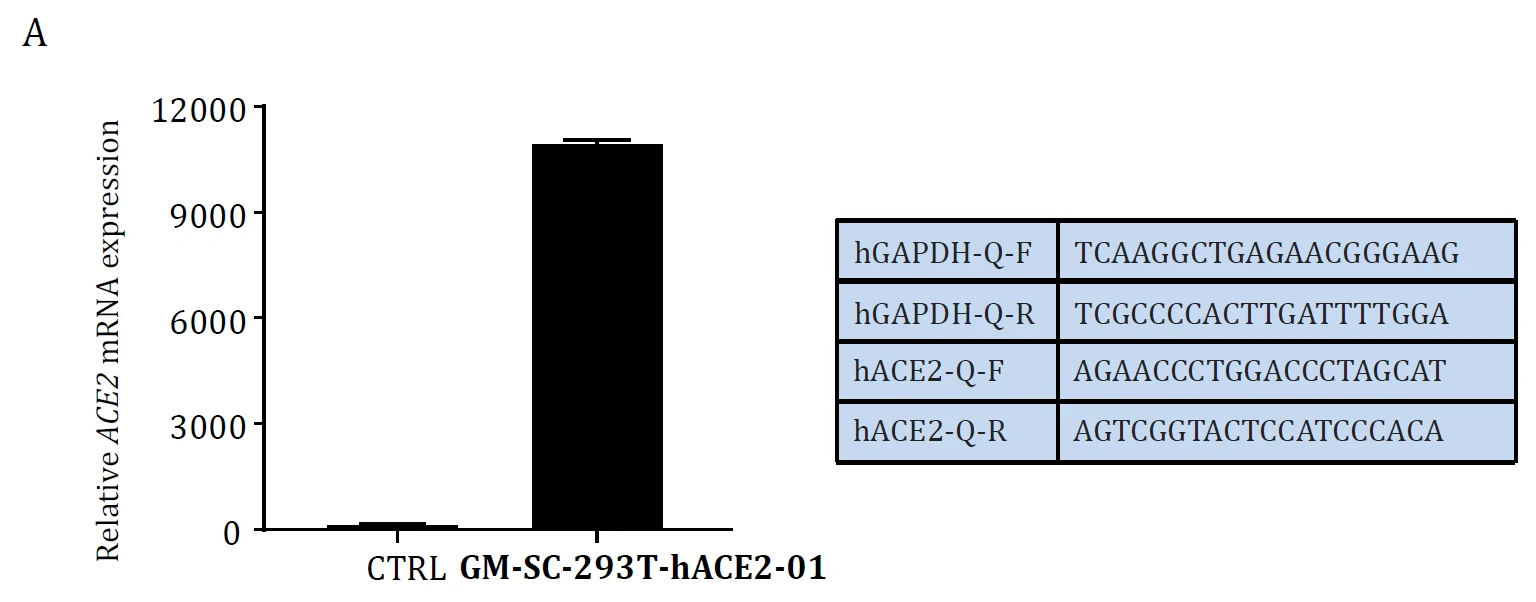
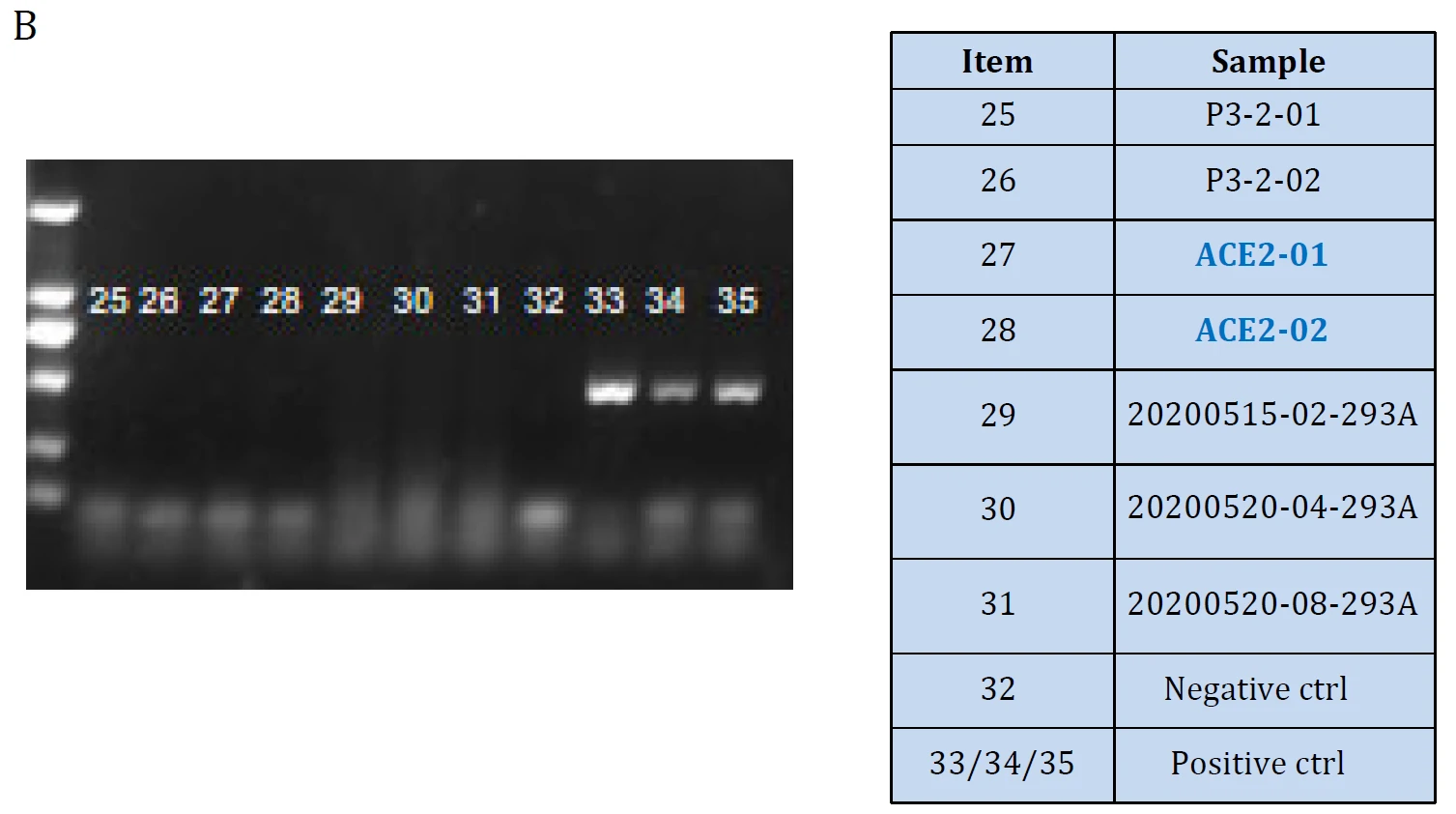
Figure. ACE2 mRNA level Validation in hACE2 overexpression stable HEK293T cell lines: Cat. GM‐SC‐293T‐hACE2‐01 (A) and the cell lines were tested as Mycoplasma free (B).
SARS-CoV-2 Pseudotyped virus
GeneMedi’s SARS-CoV-2 Pseudovirus-Luciferase (Cat.GM-2019nCoV-PSV01) is recombinant pseudotyped lentiviral particles containing SARS-CoV-2 spike protein to mimic SARS-CoV-2 (2019nCoV) cell infection and cell entry4.
The SARS-CoV-2 pseudovirus particles encode firefly luciferase and RFP in their lentiviral vector genome. The firefly luciferase and RFP gene will be strongly expressed after the SARS-CoV-2 pseudovirus entry into ACE2-expressing cells. Actually, 293T-hACE2(human ACE2 overexpression stable HEK293T cell lines) is normally used as effector cell.
GM-2019nCoV-PSV01 is a powerful tool for SARS-CoV-2 related vaccine efficacy evaluation, neutralizing antibodies, peptides blockers competitors neutralization assay, and tissue-specific infection determination.
GeneMedi SARS-CoV-2 Pseudovirus (PSV) Based Cell Entry
 Data Post Download
Data Post Download
 |
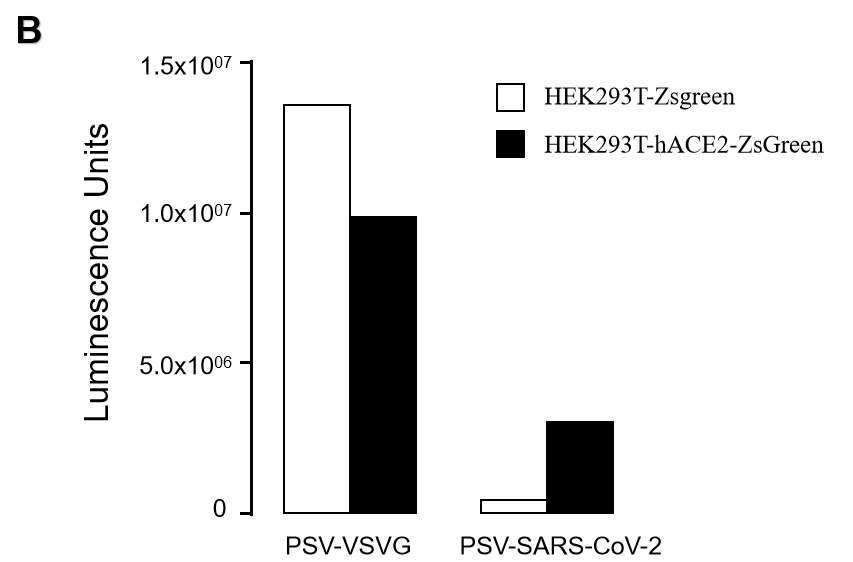 |
 |
|
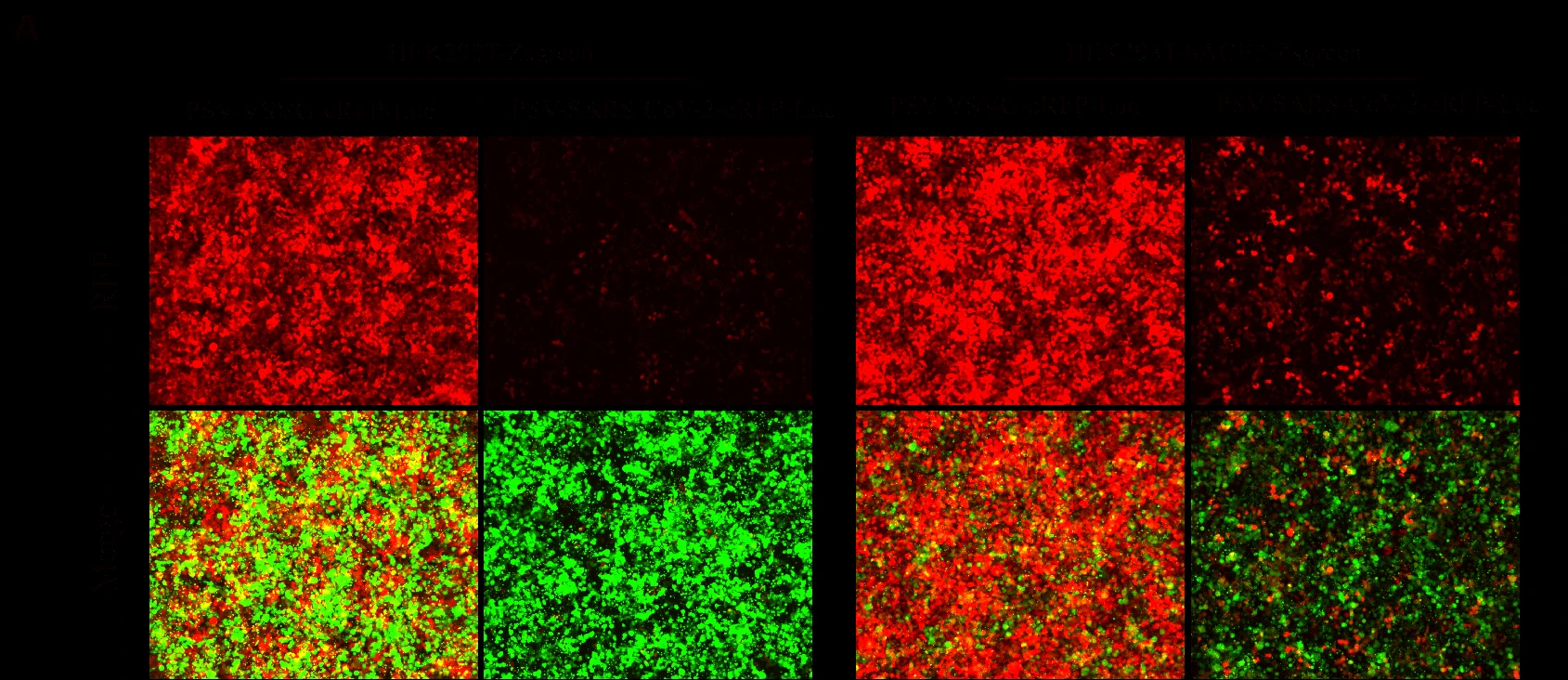
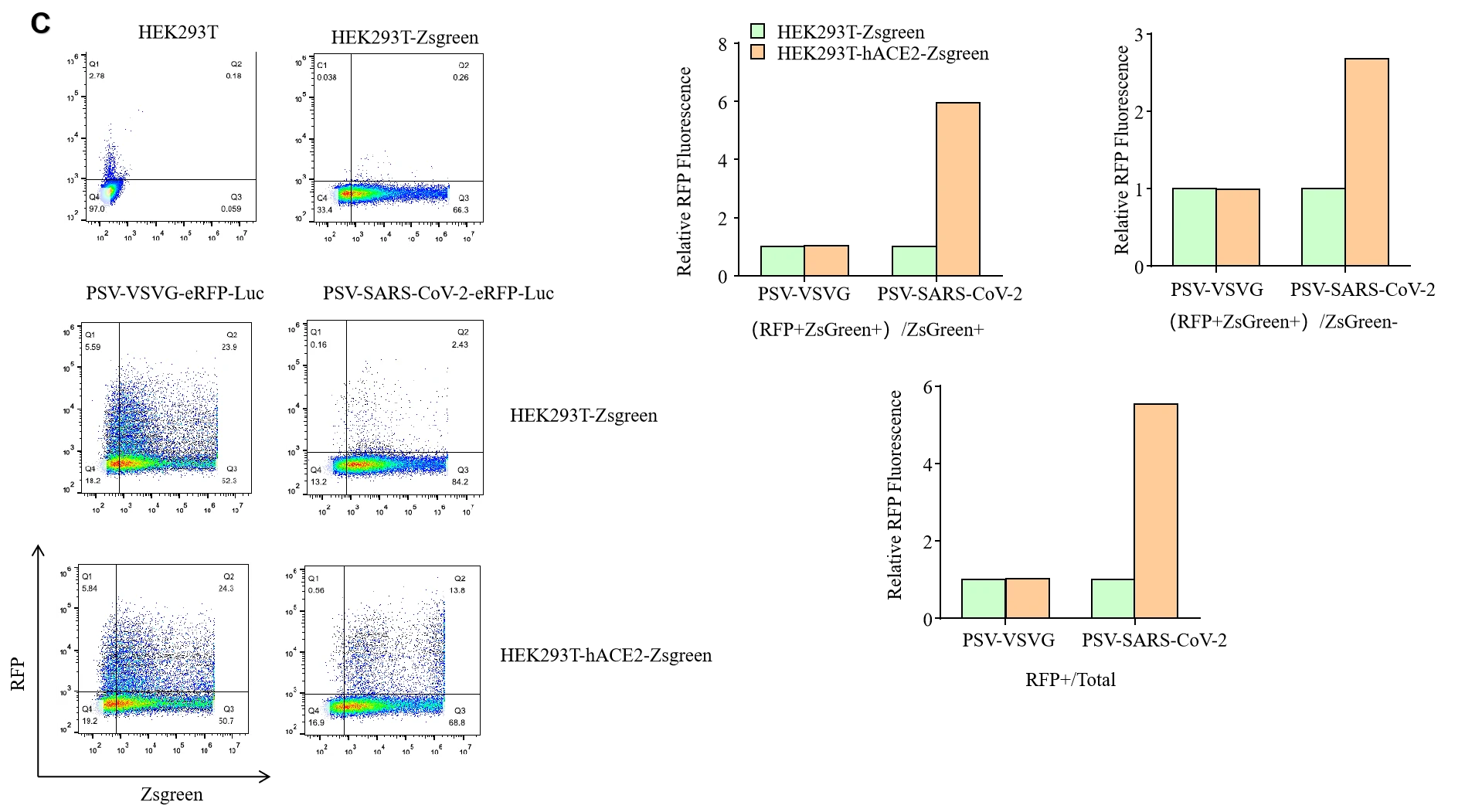
Figure. GeneMedi-SARS-CoV-2 Pseudovirus (PSV) Based Cell Entry validation with fluorescence(A), Luciferase activity (B) and FACS (C) after 72 hours of HEK293T infection. hACE2 significantly enhanced the infection efficiency of the SARS-CoV-2 PSV. GeneMedi SARS-CoV-2 PSV-Luciferase (Cat.GM-2019nCoV-PSV01) is recombinant pseudotyped lentiviral particles containing SARS-CoV-2 spike protein to mimic SARS-CoV-2 (2019nCoV) cell infection. GM-2019nCoV-PSV01 is a powerful tool for SARS-CoV-2 related vaccine efficacy evaluation, neutralizing antibodies, peptides blockers competitors neutralization assay, and tissue-specific infection determination. |
GeneMedi offers:
1. SARS-CoV-2 Pseudotyped virus packaging and production (Cat.GM-2019nCoV-PSV01)
| Catalot Number | Size(T,Test, formalized in 96 well, one test per well) |
Price(In USD) |
| GM-2019nCoV-PSV01-1 | 100T | $3,840.00 |
| GM-2019nCoV-PSV01-1 | 200T | $5,440.00 |
| GM-2019nCoV-PSV01-2 | 1000T | $21,760.00 |
| GM-2019nCoV-PSV01-2 | 5000T | $87,040.00 |
| For larger scale | Click for inquiry. | Click for inquiry. |
| Shipping & handling fee | $760.00 | |
Click here FOR INQUIRY
3. SARS-CoV-2(2019nCoV) Pseudotyped Virus Based Neutralization Assay Service for evaluating:
1) Neutralizing antibodies
2) Peptides blockers(peptide inhibitors)
3) Types of Vaccines3(by testing immunized serum from mouse, NHP etc.)
4) Compounds targeting Spike induced cell-fusion.
For GM-SC-293T-hACE2-01 & PSV service inquiry please click here to contact us or send email to [email protected]
SARS-CoV-2(2019nCoV) Pseudotyped Virus Based Neutralization Assay
In the Pseudovirus Based Neutralization Assay (PBNA), the inhibition of viral entry into cells by neutralizing antibodies is correlated to the decreased levels of firefly luciferase signals in the ACE2 expression cells (hACE2-HEK293T).
1) Neutralizing antibodies
2) Peptides blockers (peptide inhibitors)
3) Types of Vaccines3
4) Compounds targeting Spike induced cell-fusion.
Related products
References
1 XiaolongCai. An Insight of comparison between COVID-19 (2019-nCoV) and SARS-CoV in pathology and pathogenesis. Preprint, doi:10.31219/osf.io/hw34x (2020, paper of GeneMedi).2 Jean K. Millet1, Tiffany Tang3, Lakshmi Nathan3, Javier A. Jaimes4, Hung-Lun Hsu3,5, & Susan Daniel3, G. R. W. Production of Pseudotyped Particles to Study Highly Pathogenic Coronaviruses in a Biosafety Level 2 Setting. J Vis Exp, doi:10.3791/59010 (2019).
3 HuajunBai, X., XiaolongCai. Vaccines And Advanced Vaccines: -A landscape for advanced vaccine technology against infectious disease, COVID-19 and tumor. Preprint, doi:10.31219/osf.io/ypgx4 (2020, paper of GeneMedi).
4 Nie, J. et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect 9, 680-686, doi:10.1080/22221751.2020.1743767 (2020).







 Facebook
Facebook LinkedIn
LinkedIn Twitter
Twitter
