A dual-AAV approach rescues auditory function in deaf otoferlin knock-out mice
Introduction: Around one in 1000 newborns are suffered from congenital disabling hearing loss, among which, mutations in OTOF gene (encoding otoferlin protein) accounting for 2.3–10% of patients, and over one thousand pathogenic mutations have been identified within this gene. Therefore, a postnatal supplement of otoferlin cDNA into the inner ear might be a promising and effective means to ameliorate hearing loss. Recently, a dual-AAV2/6 vector, each containing half of the otoferlin cDNA, has been designed by one research team at the University Medical Center Göttingen, to treat mice suffering from deafness due to Otof depletion. The dual-AAV2/6 half-vectors were transduced into the cochleae of 6- to 7-day-old otoferlin knock-out (Otof-/-) mice, leading to restore of fast exocytosis and partially rescue of auditory function.
On December 3rd, the result was published in the journal EMBO Molecular Medicine by " A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice". The study reveals a promising and effective means to ameliorate hearing loss, and proposes a gene therapy mediated by dual-AAV vectors to treat deafness caused by mutations in large genes such as Otof. Considering the limited AAV cargo capacity of approximately 4.7–5 kb, it is difficult to transfer the 6 kb-long otoferlin cDNA into transgenic animals. Specifically, in this study, taking advantage of the dual‐AAV trans‐splicing and the hybrid strategy, the scientists first carefully generated the dual-AAV2/6 vectors. Both strategies can bring about re‐assembly of the two otoferlin cDNA fragments and expression of full‐length otoferlin.
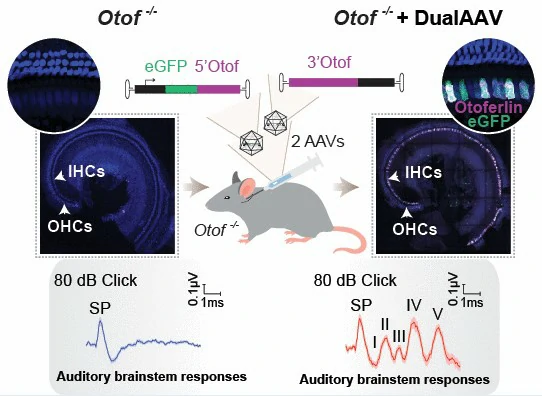 In a word, this paper proposes a gene therapy mediated by dual‐AAV vectors to treat deafness caused by OTOF gene mutations, suggesting that dual-AAV vector systems are suitable for the delivery of large transgenes into transgenic mice, which may pave the way for future gene replacement therapies of recessively inherited deafness forms like DFNB9.
In a word, this paper proposes a gene therapy mediated by dual‐AAV vectors to treat deafness caused by OTOF gene mutations, suggesting that dual-AAV vector systems are suitable for the delivery of large transgenes into transgenic mice, which may pave the way for future gene replacement therapies of recessively inherited deafness forms like DFNB9. References
1. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse
2. A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice






