Custom AAV Production Service for Gene Therapy
Order Information
-
Step 1:
Enter gene information -
Step 2:
AAV vector construction -
Step 3:
AAV packaging -
Step 4:
Custom Order confirmation
| Catalog No. | Product | Lead time(workday) |
|---|---|---|
| Buy | AAV Vector Production Scale | Final Deliverables | Lead Time(workday) |
|---|
Introduction to Custom Adeno Associated Virus (AAV) Production Service
Adeno associated virus (AAV) is one kind of human parvovirus. Recombinant AAV vectors can efficiently transfect various cell types, including dividing and quiescent cells, and induce persistent gene expression in vivo without integrating into host genome and causing any disease. These features make AAV an attractive candidate in the application of gene delivery for gene therapy and human disease model establishment. To date, AAV has been proved as the most excellent gene therapy vector. Over 204 clinical trials have been carried out using AAV vectors for gene delivery, and promising gene therapy outcomes have been achieved from clinical trials for a great number of diseases.
Genemedi has launched a comprehensive AAV production service. More than 12 AAV serotypes and a variety of capsid engineered AAV vectors are available for targeting different tissues and organs.
The engineering AAV serotypes with modification of AAV capsids contain AAV PHP.B, AAV-PHP.eB, AAV PHP.s, AAV-retro, AAV-Anc80 (L65), AAV DJ, AAVDJ8, and some other specific AAV serotypes not mentioned.
| AAV SErotype | Tissue tropism | |||||||
| CNS | Retina | Lung | Liver | Pancreas | Kidney | Heart | Muscle | |
| AAV1 | √ | √ | √ | √ | √ | |||
| AAV2 | √ | √ | √ | |||||
| AAV3 | √ | √ | √ | √ | ||||
| AAV4 | √ | √ | √ | |||||
| AAV5 | √ | √ | √ | √ | ||||
| AAV6 | √ | √ | √ | √ | √ | |||
| AAV7 | √ | √ | ||||||
| AAV8 | √ | √ | √ | √ | ||||
| AAV9 | √ | √ | √ | √ | √ | |||
| AAV-DJ | √ | √ | √ | √ | ||||
| AAV-DJ/8 | √ | √ | √ | |||||
| AAV-Rh10 | √ | √ | √ | √ | √ | |||
| AAV-retro | √ | √ | √ | |||||
| AAV-PHP.B | √ | √ | √ | |||||
| AAV8-PHP.eB | √ | √ | ||||||
| AAV-PHP.S | √ | √ | √ | |||||
Properties
| Genemedi AAV Particles | |
|---|---|
| Quantity/Unit | Vials |
| Form | Frozen form |
| Suitable Types of Infection | In vivo infection in animals |
| Sipping and Storage Guidelines | Shipped by dry ice, stored at -80 ° C, effective for 1 year. Avoid repeatedly freezing and thawing. |
| Titer | > 1*10^12v.g/ml |
Advantages
1. Safety. The wild type Adeno Associated Virus (AAV) has not currently been known to cause disease, and further security of recombinant AAV is ensured after removal of most AAV genome elements.
2. Low immunogenicity. AAV causes a very mild immune response, lending further support to its apparent lack of pathogenicity.
3. Broad range of host and specificity targeting. AAV has the ability to infect both dividing and quiescent cells, allowing genetic material to be delivered to a highly diverse range of cell types. More than 12 AAV serotypes and a variety of capsid engineered AAV vectors can be selected according to their tissue tropisms.
4. Long-term stable expression. Long term and stable expression of genes in vivo can be mediated by AAV.
5. Stable physical properties. AAV is still alive at 60℃ and resistant to chloroform.
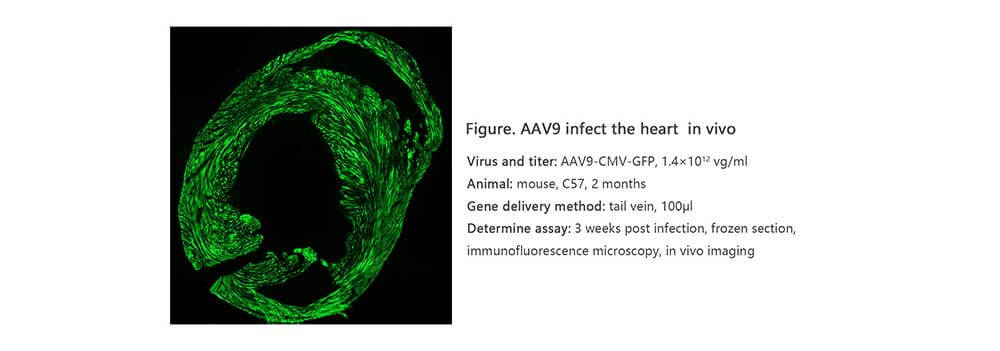
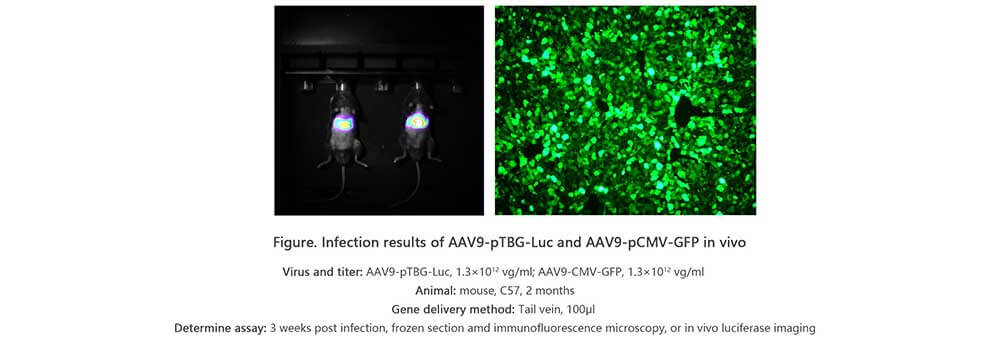
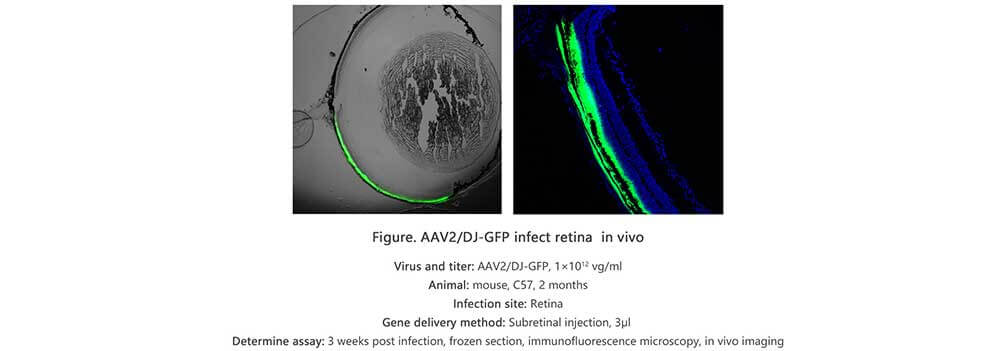
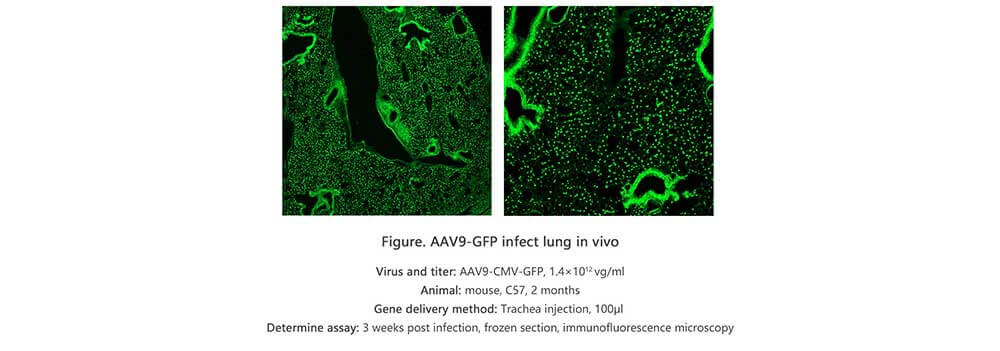
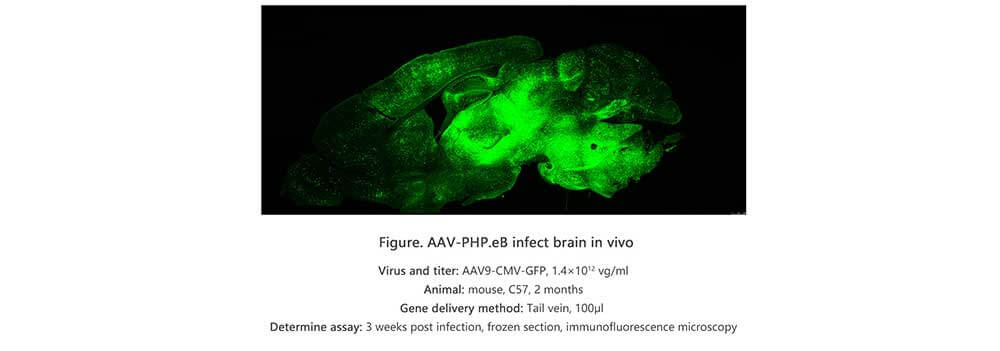
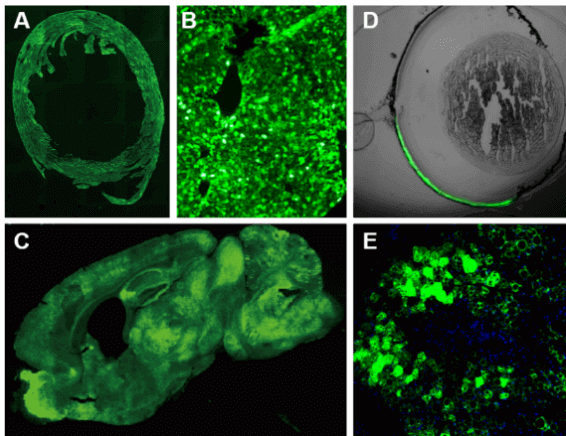
Applications and Figures
Quality control description
AAV capsid contains VR1 82kDa, VR2 72kDa and VR3 62kDa, which can be detected using the method of polyacrylamide gel electrophoresis (PAGE) followed by silver staining or Coomassie blue staining. Pure AAV should display only three major protein bands, such as shown in Fig 3.
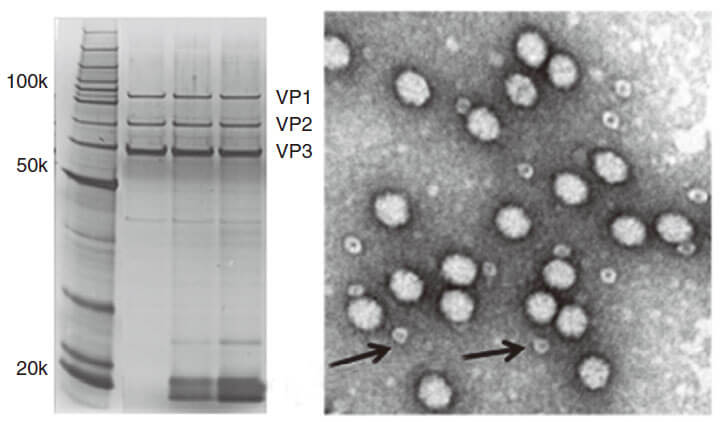 Figure 2. PAGE for quality testing example of AAV by Genemedi. Only 3 protein bands can be seen in the purified AAV virus samples.
Figure 2. PAGE for quality testing example of AAV by Genemedi. Only 3 protein bands can be seen in the purified AAV virus samples.Technical Documents
1. For further information about AAV administration and transduction results, please see  AAV(adeno associated virus) User Manual.
AAV(adeno associated virus) User Manual.
Frequently Asked Questions(FAQs)
- 1. How can I choose the optimal AAV serotypes for my in vivo study?
-
Answer
Genemedi has launched a comprehensive AAV production service. More than 12 AAV serotypes and a variety of capsid engineered AAV vectors are available for targeting different tissues and organs. Different AAV serotypes have different tissue tropism in vivo, the common serotypes and their tropism are listed in the above table 1. Therefore, you can select the most suitable AAV serotype for your study according to this table. For example, if you’d like to transfect your target gene into mouse ears, the AAV-Anc80 will be your best choice.
For your optimal AAV serotypes screening, Genemedi can also customize an AAV serotype testing kit with recommended serotypes of your choice. - 2. How much DNA do I need to provide for Custom AAV without DNA amplification?
-
Answer
You will need to provide purified plasmid DNA at a concentration of 0.5ug/ul or more.
50 ug DNA needed for Custom AAV production service without DNA amplification (10^9 GC/ml).
200 ug DNA needed for Custom AAV production service without DNA amplification (10^12 GC/ml).
300 ug DNA needed for Custom AAV production service without DNA amplification (10^13 GC/ml). - 3. What's the difference of AAV titer unit between GC/ml and vg/ml?
-
AnswerThe AAV titer unit GC/ml and vg/ml can be used interchangeably, based on qPCR method.
- 4. Why can’t I see the expression of GFP after AAV infection in my cell line?
-
Answer
AAV serotypes selection is an important parameter which may affect the transduction ability of AAV particles. Thus, it is necessary to determine which serotype works best for your cell line. For instance, serotype 5 limits its transduction ability on most cell types. For detailed information, you can refer to our technical sheet "AAV - General Guideline to Serotype Selection".
- 5. Does Genemedi’s AAV preparations belong to in-vivo grade?
-
Answer
Extensive purification steps guarantee the high quality of our viral particles, which are ready to be administrated for in-vivo models. For detailed references, please see the in-vivo infection data tested by another independent lab { www.genemedi.net }
- 6. What is the QC (quality control) method for testing AAV in Genemedi?
-
Answer
We provide qPCR-based titer as the primary method to determine whether the packaging/purification process is successful or not. If your virus has GFP or RFP reporter, we also perform virus infectivity testing in HEK293T cell line.
- 7. Can I utilize different serotypes of AAV virus in the same equipment to keep the infected cells?
-
Answer
There is no problem with using different serotypes in the same equipment, as long as the handler takes the basic precautions to avoid cross-contamination.
- 8. What is the difference between brain localization of gene expression after injection of AAV or lentiviral vectors?
-
Answer
Lentiviral particles don't spread well after stereotaxic injection into brain because the particles are relatively large (90nm~100nm). In contrast, AAV particles spread more readily due to smaller size (20nm~100nm). As mentioned, it has been stated that some AAV serotypes spread better than others in brain, for example, AAV5 is reported to spread exceptionally well when injected into striatum. Lastly, pretreatment with mannitol to your animal about 15 min ahead of viral injection has been reported to aid in spread of viral particles in the brain.
- 9. Do AAVs tranduce axons passing through a region of interest?
-
Answer
AAVs are able to infect axon terminals and produce retrograde transport (towards the cell body). The slightly longer answer is that this process is highly biased based on the serotype you are using. There are a number of papers reporting AAV6 (and maybe AAV6.2) is the main serotype to produce retrograde transport. There are however reports of many other serotypes producing retrograde transport with a much smaller rate.
Reference
1.
Li C, W Sun, C Gu, Z Yang, N Quan, J Yang, Z Shi, L Yu and H Ma. (2018). Targeting ALDH2 for Therapeutic Interventions in Chronic Pain-Related Myocardial Ischemic Susceptibility. Theranostics 8:1027-1041.
2.
Yuan Y, Y Zheng, X Zhang, Y Chen, X Wu, J Wu, Z Shen, L Jiang, L Wang, W Yang, J Luo, Z Qin, W Hu and Z Chen. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13:1754-1766.
3.
Li S, X Dou, H Ning, Q Song, W Wei, X Zhang, C Shen, J Li, C Sun and Z Song. (2017). Sirtuin 3 acts as a negative regulator of autophagy dictating hepatocyte susceptibility to lipotoxicity. Hepatology 66:936-952.
4.
Li L, B Li, M Li, C Niu, G Wang, T Li, E Krol, W Jin and JR Speakman. (2017). Brown adipocytes can display a mammary basal myoepithelial cell phenotype in vivo. Mol Metab 6:1198-1211.
5.
Feng D, B Wang, L Wang, N Abraham, K Tao, L Huang, W Shi, Y Dong and Y Qu. (2017). Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res 62.
6.
Du X, H Hao, Y Yang, S Huang, C Wang, S Gigout, R Ramli, X Li, E Jaworska, I Edwards, J Deuchars, Y Yanagawa, J Qi, B Guan, DB Jaffe, H Zhang and N Gamper. (2017). Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 127:1741-1756.
7.
Yang H, J Yang, W Xi, S Hao, B Luo, X He, L Zhu, H Lou, YQ Yu, F Xu, S Duan and H Wang. (2016). Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nat Neurosci 19:283-9.
8.
Wu X, X Wu, Y Ma, F Shao, Y Tan, T Tan, L Gu, Y Zhou, B Sun, Y Sun, X Wu and Q Xu. (2016). CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat Commun 7:13498.
9.
Wei Y, Y Chen, Y Qiu, H Zhao, G Liu, Y Zhang, Q Meng, G Wu, Y Chen, X Cai, H Wang, H Ying, B Zhou, M Liu, D Li and Q Ding. (2016). Prevention of Muscle Wasting by CRISPR/Cas9-mediated Disruption of Myostatin In vivo. Mol Ther 24:1889-1891.
10.
Zhang X, Y Yuan, L Jiang, J Zhang, J Gao, Z Shen, Y Zheng, T Deng, H Yan, W Li, WW Hou, J Lu, Y Shen, H Dai, WW Hu, Z Zhang and Z Chen. (2014). Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy 10:1801-13.