CAR-T Cell Preparation-Lentivirus Packaging Service and Products
At GeneMedi, we are dedicated to pioneering advancements in gene therapy and immunotherapy, with a particular focus on the complex yet crucial process of CAR-T cell preparation through Lentivirus packaging. The process of transferring genes in CAR-T cells is not an easy task, and there is a possibility of facing so many challenges if one will not use different vectors. The most used of them is Lentivirus (LV) because of the broad range of host specificity, and gene expression stability, and high efficiency in different mammalian cells. Our Lentivirus vectors incorporate well-designed packaging functionalities capable of holding up to 6. 5 kb of exogenous genes while having a minimal level of genotoxicity, which makes them an ideal solution for developing highly effective, strong, and stable CAR T cells. With our in-depth knowledge on Lentivirus packaging and GeneMedi advanced technologies, we strive not only to expand the effective and reliable CAR-T cell therapies, but also provide a new direction for the cancers’ treatments.
CAR Target Proteins Lentivirus Packaging Products and Services
Immunotherapy is yet another area of specialization that GeneMedi boasts of; the Lentivirus packaging services offered are highly complex and refer to the preparation of CAR-T cells every single one of which is very carefully developed to target important proteins related to different forms of cancer. Our broad range of services includes architecture, Off shoring & outsourcing solutions to the customized development of high quality lentiviral vectors for genetic modification of T cells to become the cancer cell killers. Our proposed CAR-T cell preparation services intensify the focus of antigens such as CD19, CD20, Her2, BCMA and GPC3 to provide not only the solution that cancer research and treatment strategies require, but the solutions that they demand.
Lentiviral packaging at GPI traverses the current strategies in gene therapy and empowers researchers to create CAR-T cells with effectiveness and accuracy. The dedication of GeneMedi to innovation is highlighted by use of up-to-date vector constructs, that possess the capacity to provide maximum CAR expression, fully developed T cell enlargement and prominent and persistent anti-tumor effectivity. We make a point of guaranteeing that our vectors are of the best quality and safe to use in the treatment of some of the toughest cancer ailments that practitioners and researchers may come across. It is our vision to drive the development of more cancer treatments moving them from the research stage to preclinical studies, from these to clinical trials, and finally into usage with the help of the solid Lentivirus packaging services.
| Products name | Products description | Detail |
| CD19 CAR Lentivirus | Our CD19 CAR Lentivirus is specifically designed to target CD19, a critical marker of B-cell malignancies. This vector is a cornerstone in the treatment of various leukemias and lymphomas, offering a beacon of hope for patients battling these cancers. | Details |
| CD20 CAR Lentivirus | Targeting CD20-positive malignancies, our CD20 CAR Lentivirus is engineered to facilitate groundbreaking treatments against conditions such as non-Hodgkin lymphoma and chronic lymphocytic leukemia, showcasing our commitment to combating hematological cancers. | Details |
| Her2 CAR Lentivirus | Our Her2 CAR Lentivirus targets the Her2 protein, offering a strategic approach to treating Her2-positive breast cancer. This vector embodies our dedication to advancing precision medicine and providing targeted therapies for patients with breast cancer. | Details |
| BCMA CAR Lentivirus | Focused on BCMA, a protein implicated in the pathogenesis of multiple myeloma, our BCMA CAR Lentivirus underscores our commitment to addressing this challenging condition with targeted therapeutic strategies. | Details |
| GPC3 CAR Lentivirus | By targeting GPC3, associated with hepatocellular carcinoma, our GPC3 CAR Lentivirus reflects our dedication to pioneering treatments for hard-to-treat cancers, emphasizing our innovative approach to cancer therapy. | Details |
In addition to these specialized services, GeneMedi is equipped to offer tailored Lentivirus packaging solutions, designed to meet the specific objectives of your research or therapeutic projects.
CAR Detection Tools
The GeneMedi still aims to supply the superior CAR-T cell preparation service together with a plethora of valuable CAR detection kits, which are fundamental for accurate enumeration and comprehensive investigation of CAR-T cell therapies. The assortment presented would provide both researchers and clinicians with a full spectrum of reagents and antibodies necessary to characterize CAR-T cells at each step of the process.
For the proper detection of These CAR-T cell treatments, the GeneMedi portfolio features antibodies specific to different parts of the CAR construct; scFv, hinge, transmembrane and the intracellular signal domains. It is also presupposed that with the help of this range, possible changes in CAR expression, assembly, and function can be studied comprehensively to understand how the CAR-T cells behave after infusion and engage tumor cells.
To directly detect CAR expression, the antibodies employed in this method are designed to specifically bind novel epitopes that are part of the scFv of the CAR, allowing assessment of CAR-T cell numbers in patients’ circulation. This is important for the overall assessment of CAR-T cells and their sustainability after the treatment, which gives an indication of the success of the particular therapy type.
| Cat No | Products name | Products description | Detail |
| GMLS-Tag003-Ab01 | Anti-G4S Linker monoclonal antibody | This antibody is expertly crafted to detect the G4S linker within CAR constructs, providing a reliable tool for monitoring CAR expression and ensuring the efficacy of CAR-T cell therapies. | Details |
| GTU-ADA-FMC63-Ab01~06 | Anti-FMC63 monoclonal antibody | Specifically targeting the FMC63 scFv used in CD19 CAR constructs, this antibody plays a crucial role in assessing the specificity and functionality of CD19-directed CAR-T cells, highlighting our focus on delivering precision in CAR-T cell therapy. | Details |
| GMLS-Tag002-Ab01 | Anti-VHH monoclonal antibody | Designed to recognize VHH domains, this antibody is a pivotal tool in the detection and analysis of CAR constructs that incorporate VHH domains. It offers a highly specific and sensitive means of verifying the presence and functionality of these innovative CAR constructs. | Details |
Advantages
GeneMedi's Lentivirus packaging services for CAR-T cell preparation are distinguished by several key advantages that set us apart in the field of cellular immunotherapy:
-
Unmatched Efficiency: The Lentivirus vectors that we use are of a high standard to ensure that we achieve the highest transduction efficiency, which is necessary for genetic modification of T cells to enhance their ability to recognize tumour cells.
-
Long-Term Stability: Our Lentivirus vectors also ensure stable and constant CAR gene expression hence consistent CAR-T cell response to targeted cancer cells in recurrent therapeutic application is critical to making a permanent transform.
-
Wide Applicability: The Purity of Lentivirus vectors and target cells are known to be high, this makes it possible to use Lentivirus vectors in various types of cells. This versatility is particularly important for maintaining diverse exploring and treating capabilities across the cellular context and diseases.
-
Enhanced Safety: The lentiviral vectors by Safeful focus more on safety thus resulting in low genotoxicity, hence the preference for clinical and therapeutic uses. The quality standards, which are critically followed in the production processes of our products, guarantee their safety.
-
Customization and Support: Knowing that every client is on the way to find the most suitable reagent for the research, Max Bio offers customizable options for CAR target proteins and vectors. In addition, our skilled team works to create designs starting from vector works up to the final services to tailor our services for your research and therapeutic requirements.
Validation Data
- CD19 CAR Lentivirus
- CAR Detection Tools
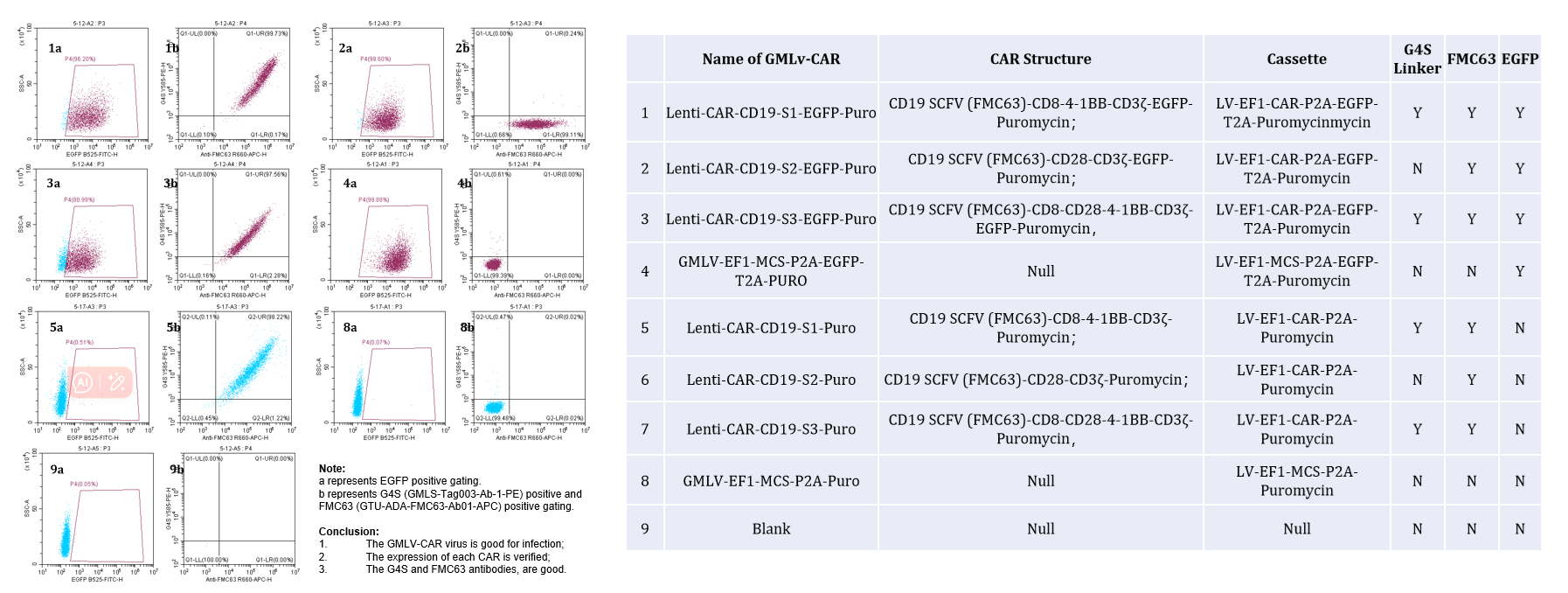
Figure 1. GM-Lenti-CAR Lentivirus represent a great performence in CAR-expression
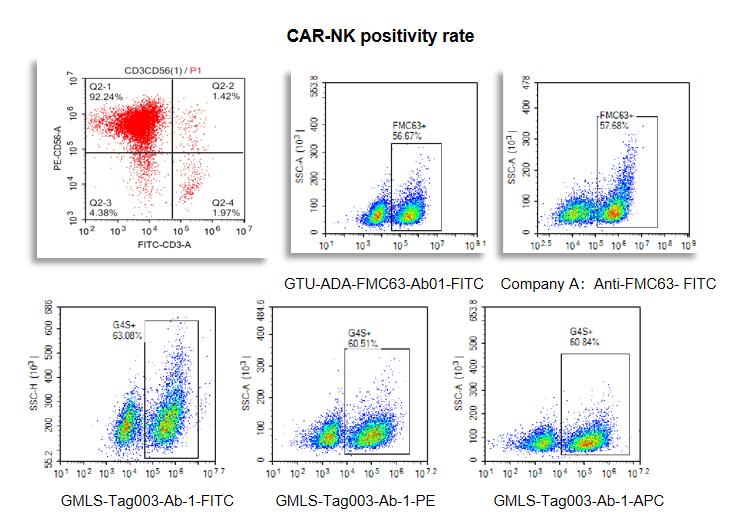
Figure 2. The GTU-ADA-FMC63-Ab01 and GMLS-Tag003-Ab-1 are validated to detect CAR-NK cells.
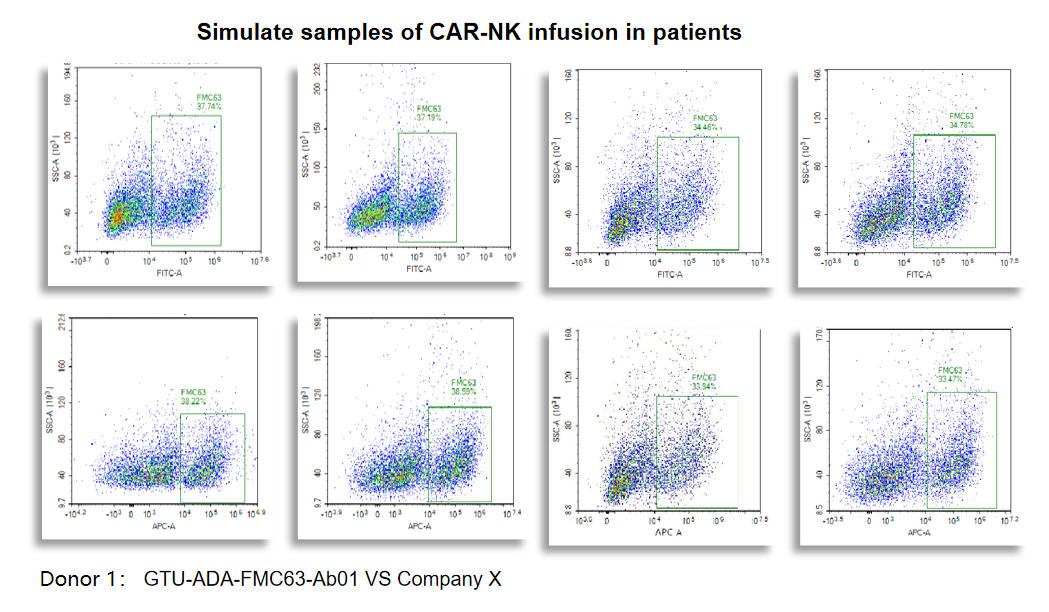
Figure 3. The GTU-ADA-FMC63-Ab01 is validated to detect Simulate samples of CAR-NK infusion in patients.
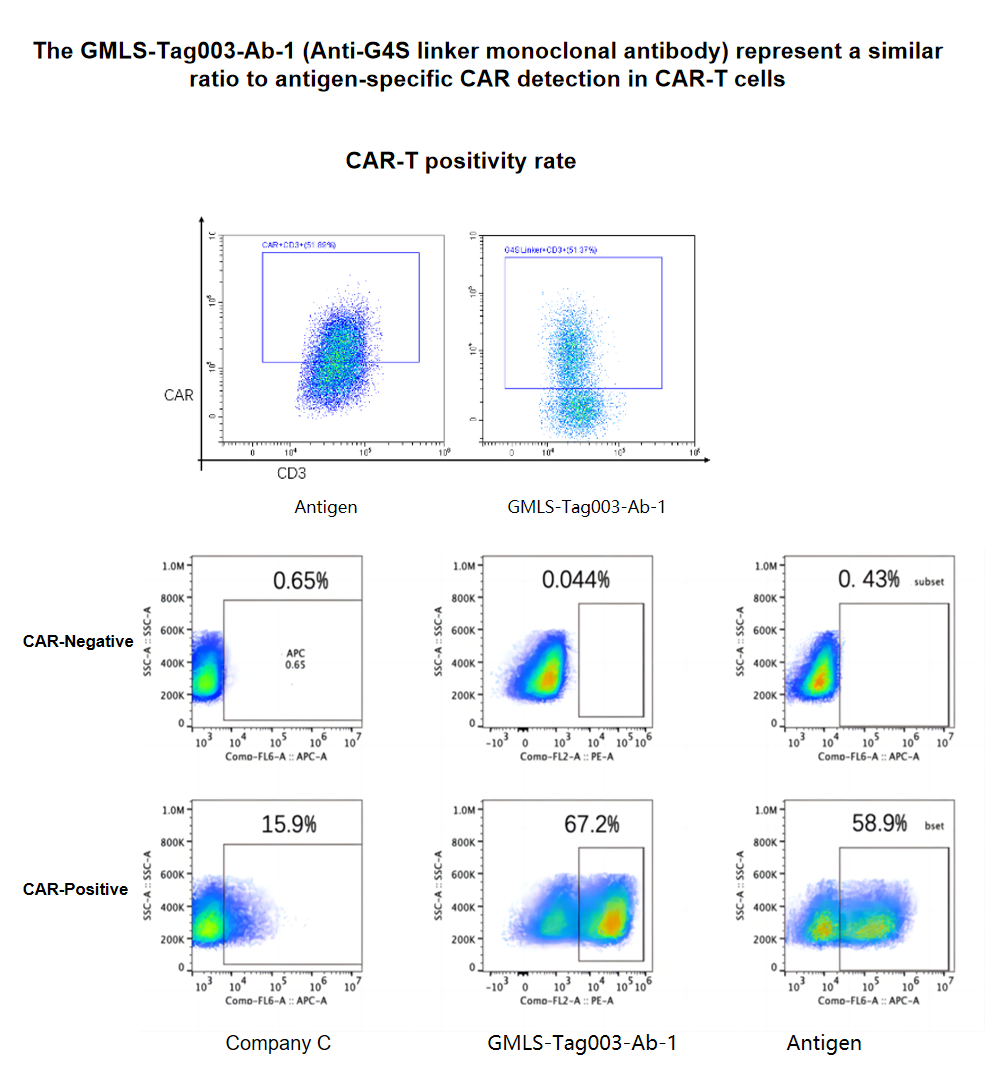
Figure 4. For detecting CAR-T cells, GeneMedi’s GMLS-Tag003-Ab-1 detection background is lower, and the detection results are closer to specific antigen detection, compared to Company C.
GMLS-Tag002-Ab01 effectively detects VHH-based format ant-Target CAR expression with great consistency with the target protein
Target protein (antigen) is usually used to detect anti-target CAR expression. For example, CD19 recombinant protein is used as a detection antigen for CAR position rate detection. However, the target protein is unique, expensive, and hard to produce. GeneMedi’s anti-VHH antibody is a universal strategy for the detection of VHH-based format anti-Target CAR expression.
293T cells were transfected with CAR plasmids expressing VHH antibodies specific to target1, target2, target3, and target4. Following transfection, the expression of the corresponding CARs was confirmed using the specific recombinant proteins, GMLS-Tag002-Ab01, and a commercial Anti-VHH antibody (Company G).
The recombinant proteins were used as positive controls to benchmark CAR-positive detection. Statistical Analysis uses the positive detection ratio of GMLS-Tag002-Ab01 or Anti-VHH antibody (Company G) relative to the antigen-based detection. GMLS-Tag002-Ab01 achieves a consistency ratio close to 1.0. The data shows that the positive detection rate with GMLS-Tag002-Ab01 was comparable to that of the antigens, demonstrating nearly identical performance to the positive control. In contrast, the anti-VHH antibody (Company G) exhibited higher or lower detection sensitivity. The results indicate that GMLS-Tag002-Ab01 effectively detects the expressed VHH antibodies (nanobody, VHH-based bispecific antibody) on CAR-transfected 293T cells with high accuracy, comparable to the recombinant proteins. This establishes GMLS-Tag002-Ab01 as a reliable tool for detecting CAR expression in similar experiments.
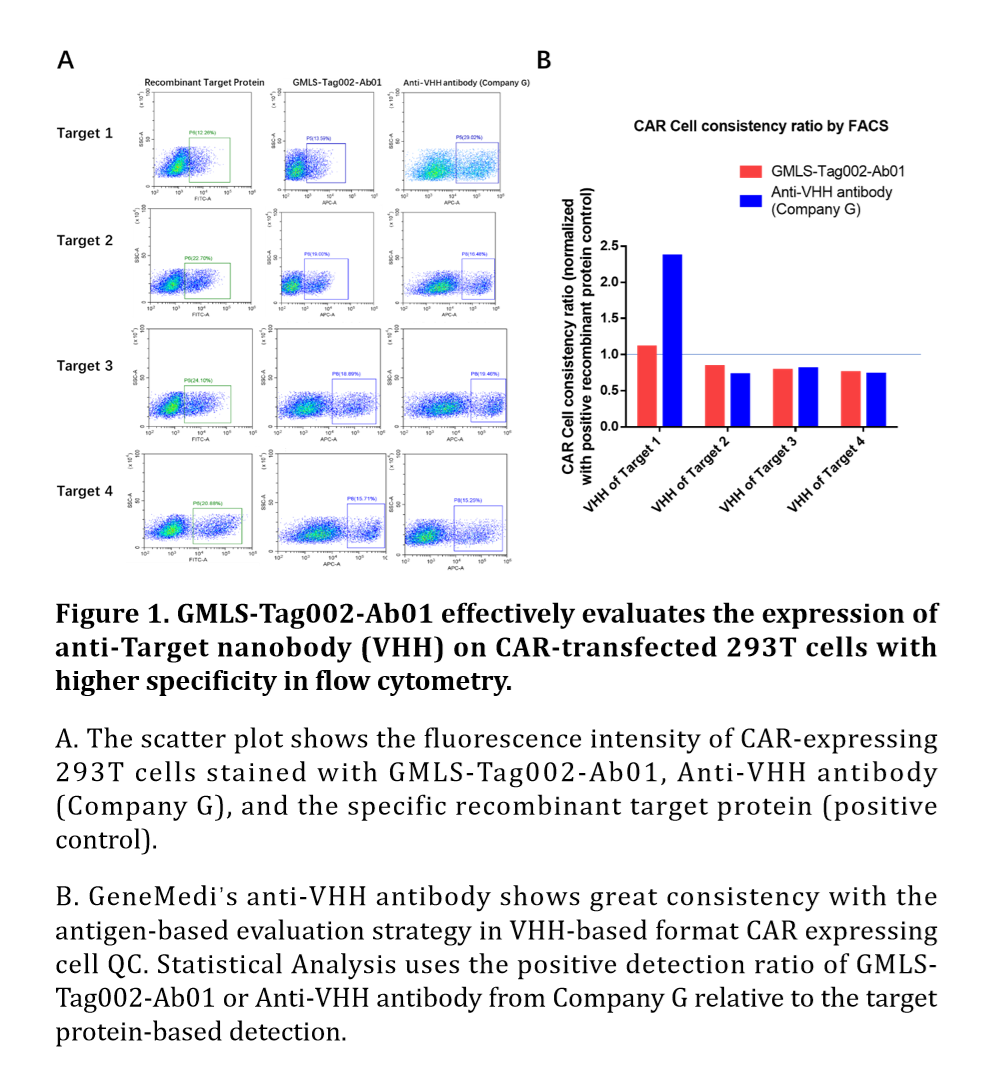
Figure 5. GMLS-Tag002-Ab01 effectively evaluates the expression of anti-Target nanobody (VHH) on CAR-transfected 293T cells with higher specificity in flow cytometry.
A. The scatter plot shows the fluorescence intensity of CAR-expressing 293T cells stained with GMLS-Tag002-Ab01, Anti-VHH antibody (Company G), and the specific recombinant target protein (positive control).
B. GeneMedi’s anti-VHH antibody shows great consistency with the antigen-based evaluation strategy in VHH-based format CAR expressing cell QC. Statistical Analysis uses the positive detection ratio of GMLS-Tag002-Ab01 or Anti-VHH antibody from Company G relative to the target protein-based detection.
Information
At GeneMedi, we are dedicated to pioneering advancements in gene therapy and immunotherapy, with a particular focus on the complex yet crucial process of CAR-T cell preparation through Lentivirus packaging. The process of transferring genes in CAR-T cells is not an easy task, and there is a possibility of facing so many challenges if one will not use different vectors. The most used of them is Lentivirus (LV) because of the broad range of host specificity, and gene expression stability, and high efficiency in different mammalian cells. Our Lentivirus vectors incorporate well-designed packaging functionalities capable of holding up to 6. 5 kb of exogenous genes while having a minimal level of genotoxicity, which makes them an ideal solution for developing highly effective, strong, and stable CAR T cells. With our in-depth knowledge on Lentivirus packaging and GeneMedi advanced technologies, we strive not only to expand the effective and reliable CAR-T cell therapies, but also provide a new direction for the cancers’ treatments.
The steps in the CAR-T cell cancer therapy process:
-
Leukapheresis: This process starts with leukapheresis which involves withdrawal of the patient’s blood and running it through a machine that isolates and retrieves the T cells. The rest of the blood components are recirculated back into the patients blood circulation system via the same veins.
-
Reprogramming: After that, the T cells are genetically modified in the laboratory environment in genetic engineering technology. A lentiviral vector is employed to transduce T cells in order to deploy the genetic sequence that would allow them to express CARs, which are receptors to be constructed on the surface of T cells.
-
CAR T-Cell: These genetically modified T cells will now be termed CAR-T cells to signal that they are programmed to bind to certain targets called antigens found on the surface of cancer cells. It is at this point that the CAR-T cells are activated on the identification of the cancer cells.
-
Expansion: These CAR-T cells are after that grown in the laboratory so that they can multiply in number Is. This expansion phase is important to nurture and develop millions of these clones CAR-T cells for reinjection into the same patient.
-
Conditioning: Before the patient is infused with CAR-T cells, they mustbe conditioned, typically, by giving them chemotherapy. This step aids in the conditioning of the body to get ready to accept the CAR-T cells through emptying the niches of the immune system hence improving on the proliferation and survival of the cells being introduced into the body.
-
Administration: The CAR-T cells expand through conditioning of receptors and are then infused to the patient The infused cells will target tumoral cell lines specific to a given patient. It then multiplies and forms special cells, which move around the body of the patient with the aim of eradication of these cancer cells.
-
Monitoring: After infusion, the patient requires a careful observation with a view of managing likely side effects that may be evident as well as determining the impact of the treatment. This is important for the safety of the patients over-emphasized as well as to make any possible changes to the management plan.
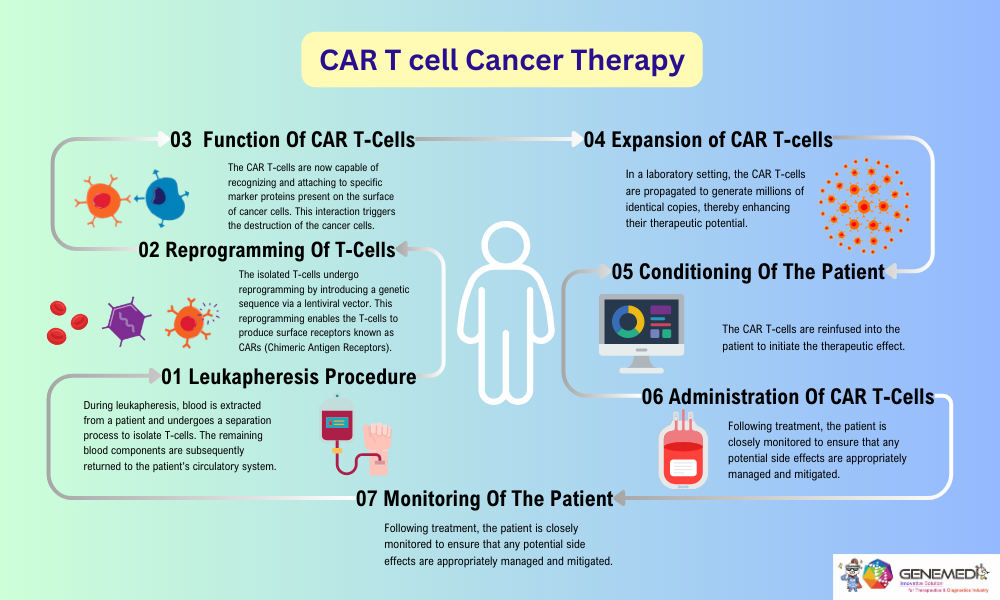
Figure 1: The entire CAR-T cell therapy process, from leukapheresis to monitoring, typically spans over 4-5 weeks. Each step is essential for the success of this personalized treatment approach, offering hope to patients with certain types of cancer.
The chimeric antigen receptors, or CARs, are engineered receptor proteins the burden a specific function onto an immune effector cell. In most conventional CARs, the domain that is charged with the responsibility of targeting consists of a single chain variable fragment (scFv), which is formed from the variable regions of both the heavy and light chains of antibodies, organized into a single binding interface.
But, advancement in this field has pushed research to create new forms of CAR constructs for antigen recognition that do not include scFvs. Some of the advanced designs, therefore, include the use of antibodies of the smallest form known as nanobodies. This is in comparison to other types of antibodies, which, while giving similar or even superior results, include disadvantages such as large size and instability.
Another type of proteins commonly employed in the current CAR constructions are, so-called designed ankyrin repeat proteins or DARPins. DARPins are small calorimetric proteins that have been used in multipurpose affinity matrices and are easy to engineer and express for the purpose of binding to a desired antigen in a selective manner.
Moreover, some CAR designs include ligands or whole receptors that ordinarily attach itself to the concerned antigen. This approach can potentially increase the stringency of the CAR-T cells and also decrease mobilisation of the CAR-T cells to other tissues.
In targeting antigen more diverse, it will create an advantage in optimizing the constructions of CAR constructs and enhance the safety and effectiveness of CAR-T cells that make them applicable in the treatment of different types of cancers.
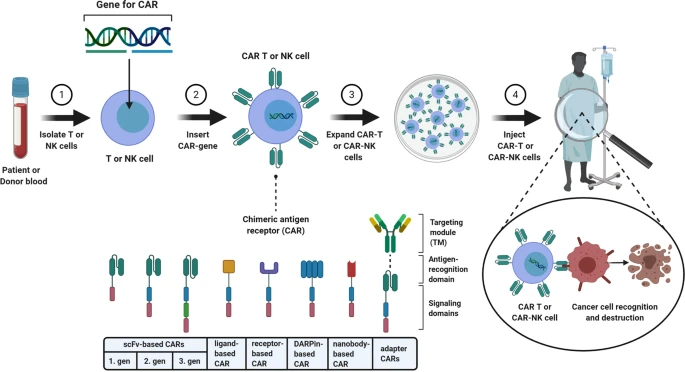
Figure 2: In most CAR constructs the targeting module consist of a single-chain variable fragment (scFv). Novel CAR constructs can also possess nanobodies, designed ankyrin repeat proteins (DARPins), ligands, or receptors instead of scFvs for target recognition.






