Pre-made Lentivirus Production Service
Order Information
Introduction to Pre-made Lentivirus Production Service
Lentivirus is a powerful tool for delivering target genes into almost all types of mammalian cells in vitro and in vivo. In contrast to retroviruses, lentiviruses are imported much more actively into the nuclei of non-dividing cells and are stably integrated into the host cells’ genome independent of cell cycle. Compared to adenovirus and adeno-associated virus (AAV), HIV-based lentiviruses are much less cell toxic, less immunogenicity, and have better transduction efficiency in many cell types, and therefore hold unique promise as gene transfer agents. Genemedi engineered transfer and packaging lentivectors offer the highest lentiviral titers available along with a wide range of selection features including a broad variety of antibiotic selection markers and fluorescent-antibiotic fusion markers for real-time transduction monitoring.
Genemedi launched more than 10 thousand transgene plasmids, which have been sequenced to guarantee the right sequence. These premade plasmid pool are available for direct lentivirus packaging and may greatly shorten the supply period of lentivirus for more than 2 weeks.
Applications for Pre-made Lentivirus:
- Delivery of expression into hard-to-transfect cell types, such as neuronal cells, without using any transfection reagents.
- Highly reproducible and controllable expression delivery method.
- Creation of stable cell lines for long-term, high-level expression.
- Expression of genes in primary or drug-arrested cells.
- Creation of transgenic animals.
- Sub-cellular localization analysis by organelle targeting.
Properties
| Lentivirus from premade plasmid | |
|---|---|
| Quantity/Unit | Vials. |
| Form | Frozen form. |
| Sipping and Storage Guidelines | Shipped by dry ice, stored at -80°C, effective for 1 year. Avoid repeatedly freezing and thawing. |
| Titer | > 1*10^8 TU/ml. |
Advantages
1. Provide a ready-to-use, easy delivery method for specific target and shrna expression.
2. Free of the often troublesome lentivector contraction and lentiviral virus production.
3. Consistent and reliable titers delivered at or above specified titer levels.
4. Fast turnaround time.
5. Safe-to-use (self-inactivating) lentiviral particles can deliver your gene into a wide range of cell lines including non-dividing, primary or stem cells.
6. Expert technical support, full confidentiality, and on-time delivery with all projects completed on-site.
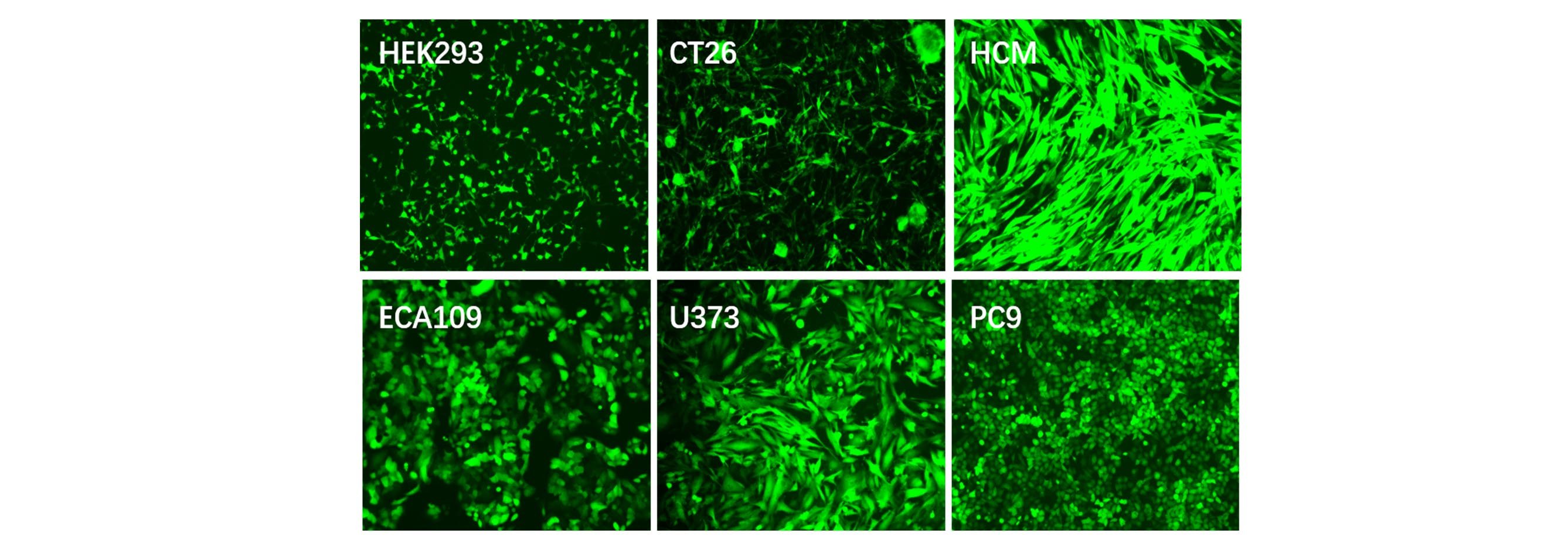
Applications and Figures
Quality control description
Our optimized production of lentiviral vector and strict quality control systems supply customers with a high titer of functional recombinant lentiviral vectors. Two methods are employed to determine viral titers: physical titer (VP/mL) and functional titer (TU/mL). Physical titer is calculated by the level of protein, such as p24, or viral nucleic acid. The functional titer, a calculation of the active virus that can infect cells, is much less than the physical titer (100-1000 fold lower). The method we adopted is functional titer, which is an accurate solution for testing virus accurate activity and MOI. The physical titer can only reflect the number of virus particles, but not reflect the true viral activity, which will cause a large error in subsequent infection experiments.
Technical Documents
1. For further information about lentivirus administration and transduction, please see  Lentivirus User Manual.
Lentivirus User Manual.
Frequently Asked Questions(FAQs)
- 1. What is a Lentivirus?
-
Answer
Lentivirus is a subfamily of the retrovirus family. Lentiviruses can deliver significant amounts of genetic information into host cells and integrate it into the cellular genome. Genetically-engineered lentiviruses are therefore used as one of the most efficient tools of gene delivery.
These lentiviruses contain a viral promoter which is used to control the expression of a transgene or shRNA but no virulence genes anymore. Together with several other security modifications this makes them safe to use in the laboratory. - 2. How does it work?
-
Answer
Co-transfection of packaging plasmids and transfer vector into a packaging cell line allows efficient production of lentiviral particles which are released into the cells supernatant.
Viral particles harvested from the cell supernatant can transduce a wide range of both dividing and non-dividing mammalian cell types. Upon infection with lentiviral particles, the single stranded RNA (ssRNA) is reverse-transcribed and the resulting double-stranded DNA (dsDNA) stably integrates into the genome of the host providing for long term transcription of gene of interest or shRNA.
- 3.What kinds of premade lentiviral particle does Genemedi provide?
-
Answer
Genemedi provides ready-to-use particles for shRNA expression and gene expression. Each particles contains a sequence fully verified shRNA or a specific gene target. Particles provided in either DMEM medium containing 10% FBS, or in PBS solution. Purified viruses in PBS are provided as in vivo ready status, suitable for in vivo applications, or for suspension cell transduction, and for transduction in cell lines requiring a serum-free culture conditions. Genemedi provides particles containing different antibiotic markers, including: Blasticidin (Bsd), puromycin (Puro), luciferase (Luc), neomycin (Neo), and fusion dual markers as: Bsd-GFP, Bsd-RFP, Puro- GFP, Puro-RFP and others. All Genemedi’s Lentiviral particles can be used for constitutive target (or shRNA) over-expression. Optionally, the same particles can be used as tetracycline inducible expression when a tetracycline regulator (tetR) protein is present (called optional inducible expression).
- 4. What's the optimal concentration of viruses that I should use for infection?
-
Answer
It depends on the purpose of the experiment. Higher titer of lentivirus should be used for in vivo experiment compared to in vitro experiment. Though lentiviral vector is highly efficient in transduction, the transduction efficiency of lentivirus depends heavily on the cell type to be transduced. Pilot experiment is highly recommended to determine how efficient the lentivirus on your target cells.
- 5. How much culture media should I use during infection?
-
Answer
For your reference, we recommend the following amount of virus-containing media for infection:
a) 10-cm plate: 8-10 ml/plate
b) 6-well plate: 1 ml/well
c) 12-well plate: 0.5 ml/well
d) 24-well plate: 0.2 ml/well - 6. How do I determine the correct amount of lentivirus to add to the target cells?
-
Answer
One of the key factors of a successful transduction is the cell type. For example, transduction efficiency is much higher in actively dividing cells than in non-dividing cells. In addition, transduction of cells works better at lower MOI (multiplicity of infection) than at higher MOI. MOI is the ratio of the number of lentivirus particles to the number of cells. For some cell types, the higher the MOI , the larger the volume and higher the titer of lentivirus is required in order for the experiment to succeed. You can adjust the cell number and add the appropriate amount of lentivirus according to what has been reported in the scientific literature. If there is no adequate information in the scientific literature, we recommend performing a preliminary experiment using gradient dilutions of lentivirus, such as 0.1 μl, 0.3 μl, 0.5 μl, 0.7 μl, 0.9 μl for Genemedi purified particles. Another important consideration for getting good transduction efficiency is the cell status. Transduction efficiency varies greatly between healthy cells and unhealthy cells. Therefore, it is essential to keep the cells as healthy as possible. For some cells with high MOI, you could also include additives such as polybrene to enhance the transduction efficiency. However, the overall health of the cells itself is always the most essential element.
- 7. Does freeze-thaw cycle infect the titer of lentiviruses
-
Answer
Yes, multiple freeze-thaw cycles may reduce the functional titer of the virus stock by up to 2-4 folds. However, first one freeze-thaw cycle does not lower the lentiviral titer. Genemedi recommends our customer not to use lentivirus that has gone through more than two freeze-thaw cycles for titer reason.
- 8. Why do my cells die in quantity after adding lentivirus to the culture medium?
-
Answer
Lentivirus has some level of toxicity to cells. It may cause damage to your cell of interest with either superfluous amounts of lentivirus, or if the infection were allowed to go on for too long a period of time. In these cases, you can adjust the multiplicity of infection (MOI) to a lower range. We recommend replacing the old culture medium with fresh complete medium 4-8 hours post transduction (no later than 12 hours post transduction).
- 9. What is the capacity of lentiviral vector as an expression system?
-
AnswerThe cloning capacity for the transgene is approximately 3-4 kb for most vector formats.
- 10. How do I create a stable cell line using lentivirus?
-
AnswerLentiviruses can stably integrate into the host cell’s genome and obtain a consistent level of expression. With a selectable marker in the lentiviral gene transfer vector plasmid, it is easy to generate a stable cell line using drug selection. You can use qRT-PCR, western blot or other detection methods to estimate the expression level of your gene.
- 11. How custom lentivirus production service proceeds:
-
Answer
If lentiviral construct is ready, please provide us:
a) The construct map or sequence.
b) 1~5 µg of your lentiviral construct plasmid DNA or bacterial culture.
We will prepare Endotoxin-free plasmid and transfect our packaging cell line for your lentivirus production. Lentiviral particles will be harvested followed by concentration and titration.
For 200 ul of lentivirus production, you will receive eight 25 µl aliquots.If you need us to generate lentiviral transfer vector for your target gene, please:
a) Choose lentiviral vector format from our vector collection and specify the gene of interest.
b) Please send us 1~5 µg plasmid or bacterial culture for your gene if it is available, otherwise please send us cDNA sequence.
We will subclone the gene of interest or shRNA into the chosen lentiviral vector and confirm its accuracy by sequencing. - 12. How long does the service take? Is my project too big (or too small)?
-
Answer
Get ready-to-transduce, high-quality, high titer lentiviral preparations at the production scale your project needs—even at large scales of up to 10 mL. Use your own lentivector construct or take advantage of our Custom Construct services and we’ll handle vector construction as well. We even have an ultra-high titer offering for demanding applications such as in vivo and stem cell transductions.
Reference
1. Wu, J. et al. MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. Journal of Clinical Investigation 125, 4091-4106 (2015).
2. Li, F., Li, S. & Cheng, T. TGF-β1 Promotes Osteosarcoma Cell Migration and Invasion Through the miR- 143-Versican Pathway. Cellular Physiology and Biochemistry 34, 2169-2179 (2014).
3. Liu, Z. et al. miR-451a Inhibited Cell Proliferation and Enhanced Tamoxifen Sensitive in Breast Cancer via Macrophage Migration Inhibitory Factor. BioMed Research International 2015, 207684-207684 (2015).
4. Si, L. et al. Smad4 mediated BMP2 signal is essential for the regulation of GATA4 and Nkx2.5 by affecting the histone H3 acetylation in H9c2 cells. Biochemical and Biophysical Research Communications 450, 81-86 (2014).
5. Han, H., Yang, S., Lin, S. G., Xu, C. S. & Han, Z. Effects and mechanism of downregulation of COX‑2 expression by RNA interference on proliferation and apoptosis of human breast cancer MCF‑7 cells. Molecular Medicine Reports 10, 3092-3098 (2014).
6. Zhang, G., Liu, Z., Cui, G., Wang, X. & Yang, Z. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumor Biology 35, 11137-11145 (2014).
7. Li, G. et al. CYC1 silencing sensitizes osteosarcoma cells to TRAIL-induced apoptosis. Cellular Physiology and Biochemistry 34, 2070-2080 (2014).
8. Mao, J., Lv, Z. & Zhuang, Y. MicroRNA-23a is involved in tumor necrosis factor-α induced apoptosis in mesenchymal stem cells and myocardial infarction. Experimental and Molecular Pathology 97, 23-30 (2014).
9. Liu, X. et al. Role of human pulmonary fibroblast-derived MCP-1 in cell activation and migration in experimental silicosis. Toxicology and Applied Pharmacology 288, 152-160 (2015).
10. Guan, G. et al. CXCR4-targeted near-infrared imaging allows detection of orthotopic and metastatic human osteosarcoma in a mouse model. Scientific Reports 5, 15244-15244 (2015).
11. Zhang, Y. et al. Role of high-mobility group box 1 in methamphetamine-induced activation and migration of astrocytes. Journal of Neuroinflammation 12, 156-156 (2015).
12. Zhu, T. et al. The Role of MCPIP1 in Ischemia/Reperfusion Injury-Induced HUVEC Migration and Apoptosis. Cellular Physiology and Biochemistry 37, 577-591 (2015).
13. Qian, M. et al. P50-associated COX-2 extragenic RNA (PACER) overexpression promotes proliferation and metastasis of osteosarcoma cells by activating COX-2 gene. Tumor Biology 37, 3879-3886 (2016).
14. Wu, N., Song, Y., Pang, L. & Chen, Z. CRCT1 regulated by microRNA-520 g inhibits proliferation and induces apoptosis in esophageal squamous cell cancer. Tumor Biology 37, 8271-8279 (2016).
15. Wang, Y. et al. Overexpression of Hiwi Inhibits the Growth and Migration of Chronic Myeloid Leukemia Cells. Cell Biochemistry and Biophysics 73, 117-124 (2015).
16. Niu, L. et al. RNF43 Inhibits Cancer Cell Proliferation and Could be a Potential Prognostic Factor for Human Gastric Carcinoma. Cellular Physiology and Biochemistry 36, 1835-1846 (2015).
17. Zhang, H. et al. ZC3H12D attenuated inflammation responses by reducing mRNA stability of proinflammatory genes. Molecular Immunology 67, 206-212 (2015).
18. Deng, X. et al. MiR-146b-5p Promotes Metastasis and Induces Epithelial-Mesenchymal Transition in Thyroid Cancer by Targeting ZNRF3. Cellular Physiology and Biochemistry 35, 71-82 (2015).
19. Zhang, B. et al. HSF1 Relieves Amyloid-β-Induced Cardiomyocytes Apoptosis. Cell Biochemistry and Biophysics 72, 579-587 (2015).
20. Hu, Q. et al. Periostin Mediates TGF-β-Induced Epithelial Mesenchymal Transition in Prostate Cancer Cells. Cellular Physiology and Biochemistry 36, 799-809 (2015).
21. Yang, Z. et al. CD49f Acts as an Inflammation Sensor to Regulate Differentiation, Adhesion, and Migration of Human Mesenchymal Stem Cells. Stem Cells 33, 2798-2810 (2015).
22. Wang, X. et al. MCPIP1 Regulates Alveolar Macrophage Apoptosis and Pulmonary Fibroblast Activation After in vitro Exposure to Silica. Toxicological Sciences 151, 126-138 (2016).
23. Gu, S., Ran, S., Liu, B. & Liang, J. miR-152 induces human dental pulp stem cell senescence by inhibiting SIRT7 expression. FEBS Letters 590, 1123-1131 (2016).
24. Jin, F., Qiao, C., Luan, N. & Li, H. Lentivirus-mediated PHLDA2 overexpression inhibits trophoblast proliferation, migration and invasion, and induces apoptosis. International Journal of Molecular Medicine 37, 949-957 (2016).
25. Liu, Z., Song, Y., Wan, L., Zhang, Y. & Zhou, L. Over-expression of miR-451a can enhance the sensitivity of breast cancer cells to tamoxifen by regulating 14-3-3ζ, estrogen receptor α, and autophagy. Life Sciences 149, 104-113 (2016).
26. Tian, Y. et al. MicroRNA-30a promotes chondrogenic differentiation of mesenchymal stem cells through inhibiting Delta-like 4 expression. Life Sciences 148, 220-228 (2016).
27. Xu, S. et al. MicroRNA-33 promotes the replicative senescence of mouse embryonic fibroblasts by suppressing CDK6. Biochemical and Biophysical Research Communications 473, 1064-1070 (2016).
28. Chen, H., Sun, M., Liu, J., Tong, C. & Meng, T. Silencing of Paternally Expressed Gene 10 Inhibits Trophoblast Proliferation and Invasion. PLOS ONE 10 (2015).
29. Deng, Y. et al. Repair of critical-sized bone defects with anti-miR-31-expressing bone marrow stromal stem cells and poly(glycerol sebacate) scaffolds. European Cells & Materials 27, 13-25 (2014).
30. Zheng, Y. & Xu, Z. MicroRNA-22 induces endothelial progenitor cell senescence by targeting AKT3. Cellular Physiology and Biochemistry 34, 1547-1555 (2014).
31. Yang, X. et al. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumor Biology 36, 383-391 (2015).
32. Wang, W. et al. p53/PUMA expression in human pulmonary fibroblasts mediates cell activation and migration in silicosis. Scientific Reports 5, 16900-16900 (2015).
33. Zhang, S. & Qi, Q. MTSS1 suppresses cell migration and invasion by targeting CTTN in glioblastoma. Journal of Neuro-oncology 121, 425-431 (2015).
34. Wang, P. et al. PFDN1, an indicator for colorectal cancer prognosis, enhances tumor cell proliferation and motility through cytoskeletal reorganization. Medical Oncology 32, 264-264 (2015).
35. Gu, S. et al. Human Dental Pulp Stem Cells via the NF-κB Pathway. Cellular Physiology and Biochemistry 36, 1725-1734 (2015).
36. Huang, G. et al. Clinical and therapeutic significance of sirtuin-4 expression in colorectal cancer. Oncology Reports 35, 2801-2810 (2016).
37. Yan, X., Ye, T., Hu, X., Zhao, P. & Wang, X. 58-F, a flavanone from Ophiopogon japonicus, prevents hepatocyte death by decreasing lysosomal membrane permeability. Scientific Reports 6, 27875 (2016).
38. Ding, W., Tong, Y., Zhang, X., Pan, M. & Chen, S. Study of Arsenic Sulfide in Solid Tumor Cells Reveals Regulation of Nuclear Factors of Activated T-cells by PML and p53. Scientific Reports 6, 19793-19793 (2016).