Lateral Flow Immunoassay
1. lateral Flow Immunoassay
Lateral flow immunoassay (LFIA) is a membrane-based technique for detecting specific analytes in complex samples. Advantages of LFIA are that it is relatively inexpensive to develop and manufacture, easy to use, and provides rapid results. Additionally, because LFIA does not require refrigerated storage, it is well-suited to use in developing countries, remote geographies, and settings with limited facilities. For these reasons, LFIA is seeing increased uptake for a broad range of applications.
A typical LFIA comprises several core components, all of which are mounted on an inert backing material and housed in a plastic case (either a cassette or a dipstick format) for easier handling. the first of these is a sample pad, an adsorbent pad permeated with salts and surfactants to promote analyte detection, which is where the sample is applied. this is followed by a conjugate release pad containing analyte-specific antibodies that are labeled with detection moieties such as colloidal gold or colored latex beads. after the conjugate release pad comes a porous membrane (usually nitrocellulose) where further antibodies are immobilized in one or more lines. commonly, both a test line and a control line are included on the membrane, which respectively function to capture the analyte and ensure the LFIA is performing correctly. The final component of the LFIA is an absorbent pad, which serves to keep the sample moving (via capillary action) and prevent backflow. as the sample migrates through the lfia, target accumulation gives rise to a signal that can normally be seen with the naked eye
Colloidal gold immunochromatographic assay
An immunochromatography assay (ICA) determines the presence or absence of a target analyte, such as pathogens or biomarkers. A schematic diagram of colloidal gold immunochromatographic assay is shown in Fig. In an colloidal gold immunochromatographic assay, an antibody against the antigen is labeled with gold particles. The labeled antibody captures the viral capsid and another antibody coated on the solid-phase carrier, thus the virus-antibody-colloidal gold particle combinations cause aggregation to occur and cause a color change to red indicating positive results. colloidal gold immunochromatographic assay do not require any specialized equipment and minimal training is needed to perform the test.
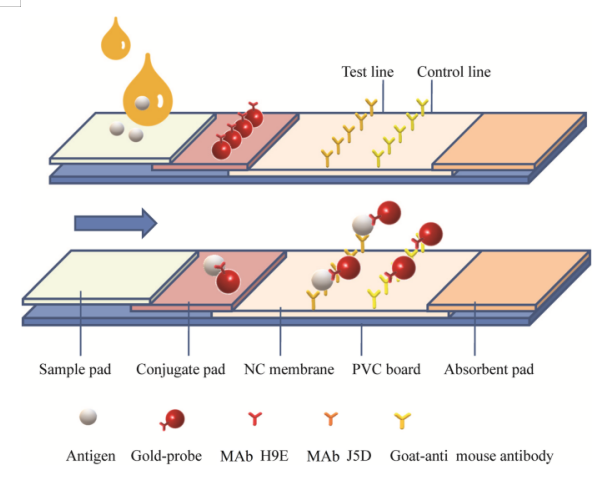
Schematic diagram of LFIA test. MAb H9E was used as labeled antibody with colloidal gold particles (in red); MAb J5D was used as capture-antibody in the T line; goat-anti mouse MAb Ig G was used in the C line. Arrow indicates the direction of the movement of antigens (capsid protein of noroviruses) (Xu, M., Lu, F., Lyu, C. et al. Broad-range and effective detection of human noroviruses by colloidal gold immunochromatographic assay based on the shell domain of the major capsid protein. BMC Microbiol 21, 22 (2021). https://doi.org/10.1186/s12866-020-02084-z)
1.1 Types of lateral flow tests
Lateral flow assays can be developed to be used in a dipstick format or in a cassette. Both dipsticks and cassette tests will work in a similar way, it is just dependent on the industry, sample matrix, and the market requirement, as to which format is suitable.
1.1.1 Sandwich format
The sandwich assay format is typically used for detecting relatively large analytes. If the analyte has at least two distinct binding sites (i.e. epitopes), a "sandwich" assay can be developed where an antibody to one epitope is conjugated to the nanoparticle and an antibody to another epitope is immobilized at the test line. The sandwich format results in a signal intensity that is proportional to the amount of analyte present in the sample.
1.1.2 Competitive format
A competitive format is used for detecting analytes in which the analyte is too small for two antibodies to bind simultaneously, such as vitamins and antibiotics. In a competitive assay, the test line contains the target analyte molecule (usually a protein-analyte complex). The nanoparticles are conjugated to an antibody that recognizes the analyte. If the analyte is not present in the sample, the nanoparticle antibody conjugates will bind to the analyte at the test line, resulting in high signal intensity. If the target analyte is present in the sample, the analyte will bind to the antibodies on the nanoparticle surface and prevent the nanoparticle from binding to the test line. This will reduce the signal at the test line resulting in a signal intensity that is inversely proportional to the amount of analyte present in the sample.
1.1.3 Multiplexed lateral flow assays
Both sandwich and competitive assays can be developed to include one or more test lines.
A multiplexed assay may be used for detecting multiple targets in a single test rather than using many individual tests. In situations where only a small sample volume is available a multiplex assay allows you to maximize its use to assist diagnosis where the presence of a number of markers together is required. It confirms the presence of multiple contaminants during high volume food and feed testing. It offers cost-saving benefits to end-users in a laboratory or in-the-field by testing for different targets simultaneously. Multiplexed testing will save time in remote or agricultural areas where resources are limited.
1.2 Application of LFIA
LFIAs can be adapted for the detection of almost any analyte. Within a healthcare setting, LFIAs are used to confirm pregnancy (e.g., by detecting the human chorionic gonadotropin hormone in urine), diagnose infection (e.g., by detecting SARS-CoV-2, the virus responsible for COVID-19, in nasopharyngeal swab samples), and establish whether a patient has an allergy (e.g., by detecting total IgE, or allergen-specific IgE, in blood). LFIAs are also being developed for numerous disease biomarkers, including prostate-specific antigen (PSA), which is often elevated by prostate cancer and other prostate disorders, and C-reactive protein (CRP), a major inflammatory marker. Outside of patient care, other applications of LFIA include screening for animal diseases, monitoring water quality, and safeguarding food production by testing for harmful contaminants such as bacterial toxins.
1.3 Advantages
- Rapid and easy-to-use test format, producing results in minutes without requiring specialized equipment or expertise
- Can be used to detect a wide range of analytes, including small molecules, proteins, and nucleic acids
- Requires minimal sample preparation, making it suitable for use in resource-limited settings or in the field
- Portable and disposable format, allowing for the potential for point-of-care testing and remote healthcare applications
- Low cost per test, making it accessible for widespread use
1.4 Disadvantages
- May have lower sensitivity and specificity compared to other immunoassay formats, such as ELISA or chemiluminescent assays
- Limited dynamic range for detecting different levels of analyte concentrations
- May not be suitable for detecting complex samples, such as those containing multiple analytes or interfering substances
- Interpretation of results may be subjective and may require interpretation by a trained operator
- Shelf life may be limited due to potential degradation of reagents over time, especially in high temperature or humid environments.
1.5 Test Procedure/ Protocol
The following is a general protocol for performing a Lateral Flow Immunoassay (LFA) test:
- Sample Collection: Collect the specimen (e.g., blood, urine, or saliva) using appropriate methods and ensure proper storage conditions.
- Sample Preparation: If required, process the sample to remove any interfering substances or concentrate the target analyte. Follow the specific instructions provided with the test kit.
- Test Kit Set-Up: Open the LFA test kit and remove all necessary components, including the test device, sample pad, conjugate pad, nitrocellulose membrane, absorbent pad, and buffer solutions. Ensure that the components are not damaged or expired.
- Application of Sample: Apply the prepared sample onto the sample pad of the test device. Sometimes, specific sample volume requirements are mentioned in the instructions. Ensure proper application and avoid overloading the sample pad with excess fluid.
- Incubation: Allow the test device to sit undisturbed at room temperature for a specified incubation period. This allows the sample to interact with the immobilized antibodies on the different regions of the test strip.
- Addition of Buffer: After the incubation period, add the buffer solution provided in the test kit. The buffer helps mobilize the sample and assists in the flow of the liquid through the membrane.
- terpretation: Observe the test lines that appear on the nitrocellulose membrane. The appearance and positioning of these lines will depend on the target analyte being tested. Different lines may represent control, test, or reference results, as indicated by the manufacturer's instructions.
- Results: Read the test results within the specified time frame mentioned in the instructions. Some LFAs provide visual cues (e.g., color changes) to indicate positive or negative results. Alternatively, certain tests may require the use of an interpretation device or reader.
- Disposal: Dispose of the used test kit components properly, following biohazard waste disposal guidelines.






 Guidence of GeneMedi's protocol / procedure for the diagnostics application:
Guidence of GeneMedi's protocol / procedure for the diagnostics application: 