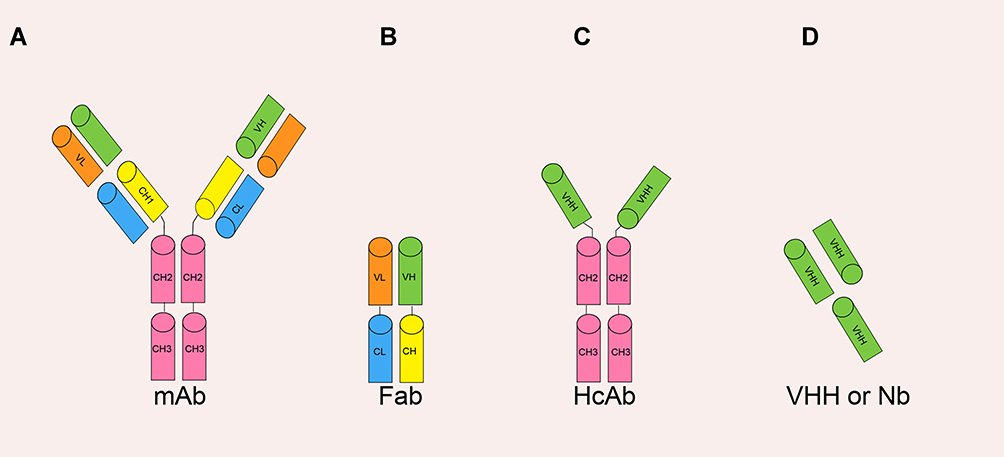
Nanobody (Nb) is the smallest natural antigen-specific binding functional fragment of about 12~15 kb (Figure 2D). The molecular weight of Nb is smaller than the monoclonal antibody mAb (~150 kb), Fab fragment (~55 kb) or HcAb (~90 kb) Figure 1A–C. Former study showed that these nanobodies can be genetically engineered from the heavy-chain antibody derived from camelids or cartilaginous fish. Their immune systems have naturally evolved into high-affinity V-like domains. Nb has a stronger and faster tissue penetration ability and can reach dense tissues such as solid tumors. The half-life of Nb in the blood is also relatively shorter due to renal filtration.
Nb consists of three antigenic complementary determining regions (complementarity determining region, CDR) and four frame regions (frame region, FR). Among them, three CDR are the binding regions of Nb to the antigen. The longer amino acid sequences of CDR1 and CDR3 of Nb make up for the loss of antigen-binding ability caused by the loss of light chain to some extent. After receiving antigen stimulation, the production of Nb mainly depends on somatic hypermutation, so a longer CDR sequence also means more antibody diversity. Crystallographic studies have shown that longer CDR3 regions give Nb a stronger ability to bind antigens. Sequence analysis disclosed that the VH domain of Nb was highly homologous to that of human immunoglobulin IgG, but there were significant differences between FR2 and CDR3 regions. Repeated administration of Nb could induce both humoral and cellular immune response.