Penicillin G/Benzylpenicillin antibody/antigen (BSA/OVA/KLH conjugated hapten) GMP-SMT-63
anti-Penicillin G/Benzylpenicillin antibody and Carrier-coupled antigen/immunogen (hapten-carrier conjugates)
 Go to Veterinary Drug residues and Food Additives diagnostics products collection
>>
Go to Veterinary Drug residues and Food Additives diagnostics products collection
>>
Product information
Size: 1mg | 10mg | 100mg
| Catalog No. | Description | US $ Price (per mg) | First Order Discount | First Order Discount Price |
|---|---|---|---|---|
| GMP-SMT-63-Ag-1 | BSA-Penicillin G/Benzylpenicillin | 756 | ||
| GMP-SMT-63-Ag-2 | OVA-Penicillin G/Benzylpenicillin | 756 | ||
| GMP-SMT-63-Ab-1 | Anti-Penicillin G/Benzylpenicillin mouse monoclonal antibody | 1953 | 20% | 1562.4 |
| GMP-SMT-63-Ab-2 | Anti-Penicillin G/Benzylpenicillin human monoclonal antibody | 1953 | 20% | 1562.4 |
| GMP-SMT-Sensor-1 | Recombinant beta-lactams sensor protein | 1953 | ||
| GMP-SMT-63-Ag-3 | BSA-Penicillin G/Benzylpenicillin | 756 | ||
| GMP-SMT-63-Ab-3 | Anti-Penicillin G/Benzylpenicillin mouse monoclonal antibody | 1953 | 20% | 1562.4 |
Shipping Costs: $360–$760
Antibodies: $360 Antigens: $760 (Elevated cost due to antigen heterogeneity, post-translational modifications, structural complexity, and specialized handling.)
Product Description
BSA-Penicillin G/Benzylpenicillin
| Cat No. of Products | GMP-SMT-63-Ag-1, GMP-SMT-63-Ag-3 |
| Product Name | BSA-Penicillin G/Benzylpenicillin |
| Expression platform | |
| Target | Penicillin G/Benzylpenicillin |
| Bioactivity validation | Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody; |
| Products description | Competitive immunoassay-validated hapten-carrier conjugates BSA-Penicillin G/Benzylpenicillin with anti-Hapten antibody. The hapten hapten-carrier conjugates BSA-Penicillin G/Benzylpenicillin had been validated with our anti-Hapten antibody Anti-Penicillin G/Benzylpenicillin mouse monoclonal antibody via competitive ELISA test. |
| Application |
ELISA tests and other immunoassays; Lateral flow immunoassay (LFIA); LTIA; Immunonephelometry; Time-resolved Fluorescence Immunoassay (TRFIA); |
| Formulation & Reconstitution | PBS liquid / Lyophilized from GM's Protein Stability Buffer2 (PSB2,Confidential Ingredients) or PBS (pH7.4); For PSB2, reconstituted with 0.9% sodium chloride; For PBS, reconstituted with ddH2O. |
| Storage | Store at -20℃ to -80℃ under sterile conditions. Avoid repeated freeze-thaw cycles. |
OVA-Penicillin G/Benzylpenicillin
| Cat No. of Products | GMP-SMT-63-Ag-2 |
| Product Name | OVA-Penicillin G/Benzylpenicillin |
| Expression platform | |
| Target | Penicillin G/Benzylpenicillin |
| Bioactivity validation | Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody; |
| Products description | Competitive immunoassay-validated hapten-carrier conjugates OVA-Penicillin G/Benzylpenicillin with anti-Hapten antibody. The hapten hapten-carrier conjugates OVA-Penicillin G/Benzylpenicillin had been validated with our anti-Hapten antibody Anti-Penicillin G/Benzylpenicillin mouse monoclonal antibody via competitive ELISA test. |
| Application |
ELISA tests and other immunoassays; Lateral flow immunoassay (LFIA); LTIA; Immunonephelometry; Time-resolved Fluorescence Immunoassay (TRFIA); |
| Formulation & Reconstitution | PBS liquid / Lyophilized from GM's Protein Stability Buffer2 (PSB2,Confidential Ingredients) or PBS (pH7.4); For PSB2, reconstituted with 0.9% sodium chloride; For PBS, reconstituted with ddH2O. |
| Storage | Store at -20℃ to -80℃ under sterile conditions. Avoid repeated freeze-thaw cycles. |
Anti-Penicillin G/Benzylpenicillin mouse monoclonal antibody
| Cat No. of Products | GMP-SMT-63-Ab-1, GMP-SMT-63-Ab-3 |
| Product Name | Anti-Penicillin G/Benzylpenicillin mouse monoclonal antibody |
| Expression platform | |
| Target | Penicillin G/Benzylpenicillin |
| Bioactivity validation | Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody; Lateral flow immunoassay (LFIA); |
| ELISA IC50 (ppb) | 0.1-0.2 |
| Products description | The anti-Hapten antibody against hapten Penicillin G/Benzylpenicillin had been validated with our hapten hapten-carrier conjugates BSA-Penicillin G/Benzylpenicillin via competitive ELISA test. |
| Host of Antibody | Mouse IgG |
| Application |
ELISA tests and other immunoassays; Lateral flow immunoassay (LFIA); LTIA; Immunonephelometry; Time-resolved Fluorescence Immunoassay (TRFIA); |
| Formulation & Reconstitution | Lyophilized from GM's Protein Stability Buffer2 (PSB2,Confidential Ingredients) or PBS (pH7.4); For PSB2, reconstituted with 0.9% sodium chloride; For PBS, reconstituted with ddH2O. |
| Storage | Store at -20℃ to -80℃ under sterile conditions. Avoid repeated freeze-thaw cycles. |
Anti-Penicillin G/Benzylpenicillin human monoclonal antibody
| Cat No. of Products | GMP-SMT-63-Ab-2 |
| Product Name | Anti-Penicillin G/Benzylpenicillin human monoclonal antibody |
| Expression platform | |
| Target | Penicillin G/Benzylpenicillin |
| Bioactivity validation | Competitive immunoassay validation (Competitive ELISA) with hapten-carrier conjugates and anti-Hapten antibody; Lateral flow immunoassay (LFIA); |
| ELISA IC50 (ppb) | 0.1-0.2 |
| Products description | The anti-Hapten antibody against hapten Penicillin G/Benzylpenicillin had been validated with our hapten hapten-carrier conjugates BSA-Penicillin G/Benzylpenicillin via competitive ELISA test. |
| Host of Antibody | Human IgG1 |
| Application |
ELISA tests and other immunoassays; Lateral flow immunoassay (LFIA); LTIA; Immunonephelometry; Time-resolved Fluorescence Immunoassay (TRFIA); |
| Formulation & Reconstitution | Lyophilized from GM's Protein Stability Buffer2 (PSB2,Confidential Ingredients) or PBS (pH7.4); For PSB2, reconstituted with 0.9% sodium chloride; For PBS, reconstituted with ddH2O. |
| Storage | Store at -20℃ to -80℃ under sterile conditions. Avoid repeated freeze-thaw cycles. |
Recombinant beta-lactams sensor protein
| Cat No. of Products | GMP-SMT-Sensor-1 |
| Product Name | Recombinant beta-lactams sensor protein |
| Expression platform | |
| Target | Penicillin G/Benzylpenicillin |
| Bioactivity validation | |
| Products description | |
| Application |
ELISA tests and other immunoassays; Lateral flow immunoassay (LFIA); LTIA; Immunonephelometry; Time-resolved Fluorescence Immunoassay (TRFIA); |
| Formulation & Reconstitution | PBS liquid / Lyophilized from GM's Protein Stability Buffer2 (PSB2,Confidential Ingredients) or PBS (pH7.4); For PSB2, reconstituted with 0.9% sodium chloride; For PBS, reconstituted with ddH2O. |
| Storage | Store at -20℃ to -80℃ under sterile conditions. Avoid repeated freeze-thaw cycles. |
Reference
Validation Data
Application development scientists of GDU (GeneMedi Diagnostics Unit) have
generated and optimized the beta-lactams sensor protein with a larger linear range and better
sensitivity against the beta-lactams. We tested 24 kinds of beta-lactams. Below is the
competitive ELISA data of GeneMedi’s optimized beta-lactams sensor recombinant protein. For
Ampicillin EC50 = 0.53 ng/ml (ppb); For Penicillin G, EC50 = 0.19 ng/ml (ppb). Our Recombinant
beta-lactams sensor protein has obtained good performances, such as the stability and
sensitivity.
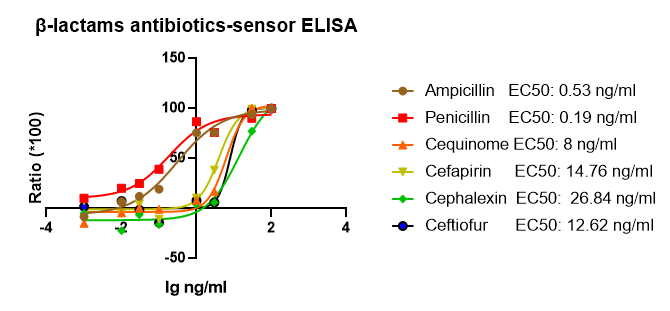
Figure 1. GeneMedi's Recombinant beta-lactams sensor protein was validated to detect the
beta-lactams (Ampicillin, Penicillin G, Cequinome, Cefapirin, Cephalexin, Ceftiofur) in
ELISA.
| Beta-lactams | Cross-Reactivity | Beta-lactams | Cross-Reactivity |
| Penicillin G | Y | Nafcillin | Y |
| Ampicillin | Y | Cephalothin | Y |
| Ceftiofur | Y | Cefapirin | Y |
| Cefquinome | Y | Cefoperazone | Y |
| Cephalosporin | Y | Cefotaxime | Y |
| Cephalexin | Y | Cefalotin | Y |
| Benzylpenicillin | Y | Cefuroxime | Y |
| Penicillin V | Y | Cefaclor | Y |
| Amoxicillin | Y | Ceftriaxone | Y |
| Oxacillin | Y | Cefamandole | Y |
| Cloxacillin | Y | Moxalactam | Y |
| Dicloxacillin | Y | Meropenem | Y |
| Kanamycin (Negative Control) | N | Tetracycline (Negative Control) | N |
Table1. GeneMedi's Recombinant beta-lactams sensor protein can detect 24 kinds of beta-lactams with high sensitivity.
Click to get more Data / Case study about the product.
About GDU

GDU helps global diagnostic partners in high quality of raw material discovery, development, and application. GDU believes in Protein&antibody Innovation for more reliable diagnostic solutions.







Comments
No comments yet.