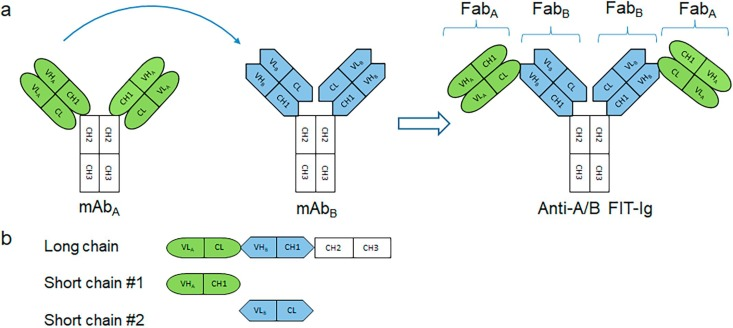
Tetravalent Fabs-In-Tandem immunoglobulins (FIT-Ig™) technology combines Fab fragments of any 2 parental mAbs create a tetravalent, dual-targeting single molecular entity, where the FabA is structurally fused to FabB in tandem at its N-terminus (Fig. 1a). A unique crisscross orientation of 2 sets of VH-CH1 and VL-CL evades any mispairing problem between two short chains and long chain. FIT-Ig have a complete Fc domain which is required for the formation of a disulfide-linked full IgG-like molecule. There is no peptide linker between two Fab moieties, no amino acid mutation in any area, and no altered Fab domain, which potentially reduce the immunogenicity risk. FIT-Ig design also provides enough flexibility allowing independent target engagement by the two antigen binding domains, as the two Fabs are connected only in one chain, instead of in both chains, therefore providing enough space for the lower domain to engage even a large antigen without any steric hindrance. FIT-Ig molecule is symmetrical and composed of long chains, 2 short chains during expression (Fig. 1b). The proper assembly is influenced by molar ratio of 3 polypeptide chains. It is known Fab domain usually have equivalent antigen specificity with mAb. FIT-Ig that utilizes 2 intact Fab domains tend to retains target binding of both parental mAbs. FIT-Ig molecule therefore can be designed based on the properties of the 2 parental mAbs.