Cre-loxp system and Viral vector (AAV and adenovirus)-based Cre tools (AAV-Cre and Ad-Cre)
Product list: Cre/loxP tools in AAV vector(AAV-Cre), adenoviral vector(Ad-Cre) and lentiviral vector(Lv-Cre)
| Cat No. | Pre-made Cre | Viral vector | Protomer | Promoter Tissue Specificity |
Promoter Tissue Specificity characteristics |
| GMV-AdCre-01 | Ad-CMV-Cre | Adenovirus | CMV | Total | Total |
| GMV-AdCre-02 | Ad-CMV-Cre-GFP | CMV | Total | Total | |
| GMV-AAV9Cre-01 | AAV9-CMV-Cre | AAV GeneMedi offer multiple serotypes of AAV-Cre loxP system for your option:
· AAV1-Cre · AAV2-Cre · AAV2 variant (Y444F)-Cre · AAV2 variant (Y272F, Y444F, Y500F, Y730F)-Cre · AAV2 variant (Y444F, Y730F, Y500F, Y272F, Y704F, Y252F)-Cre · AAV2 variant(AAV2.7m8)-Cre · AAV5-Cre · AAV6-Cre · AAV8-Cre · AAV8-1m-Cre · AAV8-2m-Cre · AAV8 variant (Y733F, Y447F, Y275)-Cre · AAV9-Cre · AAV-Rh.10-Cre · AAV-DJ-Cre · AAV-DJ/8-Cre · AAV-Retro (Retrograde)-Cre · AAV-PHP.B-Cre · AAV9-PHP.A-Cre · AAV-PHP.eB-Cre · AAV-PHP.S-Cre | CMV | Total | Total |
| GMV-AAV9Cre-02 | AAV9-CMV-Cre-ZsGreen | CMV | Total | Total | |
| GMV-AAV9Cre-03 | AAV9-CAG-Cre | CAG | Total | Total | |
| GMV-AAV9Cre-04 | AAV9-CAG-Cre-ZsGreen | CAG | Total | Total | |
| GMV-AAV9Cre-05 | AAV9-Syn-Cre-EGFP | synapsin (SYN) | Neuro | Neuron-specific promoter | |
| GMV-AAV9Cre-06 | AAV9-Syn-Cre | synapsin (SYN) | Neuro | Neuron-specific promoter | |
| GMV-AAV9Cre-07 | AAV9-CaMKII-Cre-EGFP | CaMKII | Neuro | Forebrain glutamate neuron-specific promoter | |
| GMV-AAV9Cre-08 | AAV9-CaMKII-Cre | CaMKII | Neuro | Forebrain glutamate neuron-specific promoter | |
| GMV-AAV9Cre-09 | AAV9-GFAP-Cre-EGFP | GFAP | Neuro | Astrocyte specific promoter | |
| GMV-AAV9Cre-10 | AAV9-GFAP-Cre | GFAP | Neuro | Astrocyte specific promoter | |
| GMV-AAV9Cre-11 | AAV9-cTNT-Cre | cTnT | heart | Cardiomyocyte specific promoter | |
| GMV-AAV9Cre-12 | AAV9-cTNT-Cre-EGFP | cTnT | heart | Cardiomyocyte specific promoter | |
| GMV-AAV9Cre-13 | AAV9-TBG-Cre | TBG | liver | Hepatic specific promoter | |
| GMV-AAV9Cre-14 | AAV9-TBG-Cre-ZsGreen | TBG | liver | Hepatic specific promoter | |
| GMV-AAV9Cre-15 | AAV9-TIE-Cre | TIE | endothelial | Endothelial-specific promoter | |
| GMV-AAV9Cre-16 | AAV9-cd68-Cre | cd68 | Neuro | Microglia-specific promoter | |
| GMV-AAV9Cre-17 | AAV9-c-fos-Cre | c-fos | Neuro | Excitatory neuron promoter | |
| GMV-AAV9Cre-18 | AAV9-mecp2-Cre | mecp2 | Neuro | Shorter neuron-specific promoter | |
| GMV-AAV9Cre-19 | AAV9-MHCK7-Cre | MHCK7 | muscle | Muscle specific promoter | |
| GMV-AAV9Cre-20 | AAV9-Rpe65-Cre | Rpe65 | retina | Retinal specific promoter | |
| GMV-AAV9Cre-21 | AAV9-sm22a-Cre | sm22a | muscle | Smooth muscle specific promoter | |
| GMV-AAV9Cre-22 | AAV9-Spc5-12-Cre | Spc5-12 | muscle | Myocyte specific promoter | |
| GMV-AAV9Cre-23 | AAV9-Ta1-Cre | Tal | Neuro | Early neuron-specific promoter | |
| GMV-LvCre-01 | Lv-CMV-Cre | Lentivirus | CMV | Total | Total |
| GMV-LvCre-02 | Lv-CMV-Cre-zsgreen | CMV | Total | Total |
Abstract
Cre-loxP system is widely used in the field of biosciences, especially in the generation of genetically engineered mice (knockout or overexpression), enabling researchers to study the function of gene of interest (GOI). To date, numerous systems, derived from the basic Cre-loxP system, have been developed to precisely control gene expression at desired time or cells. Here, we briefly introduce Cre-loxP system, and give a summary about its applications, especially viral vectors-mediated Cre expression (such as AAV-Cre or Ad-Cre) for in vitro or in vivo studies.Inducible-tissue specific Cre-loxP system for in vivo and in vitro study
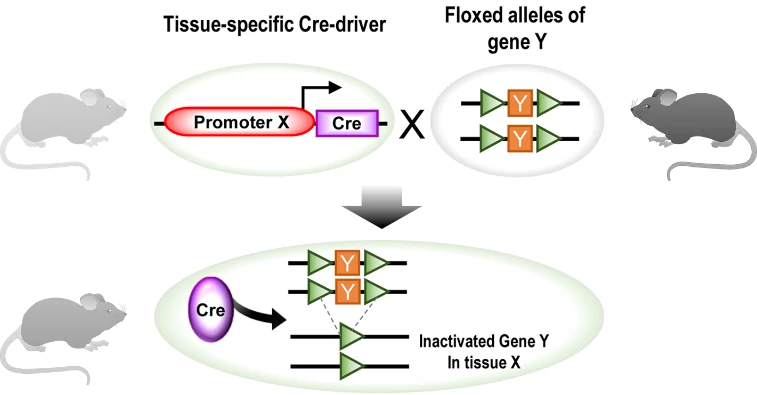
a) Tissue-specific promoter driven Cre-loxP system for conditional knock-out (KO)
To achieve the conditional knock-out (KO) of gene of interest (GOI) in specific tissue or organ, cell-specific promoter/enhancer driven Cre lines are used to breed with GOI-loxP lines. Their offspring will get both cell-specific driven Cre and floxed DNA (GOI), resulting in tissue-specific GOI excision (Fig. 3). A great number of cell-specific promoters or enhancers have been developed, such as Lyz2 driven Cre specific in macrophage, and CD45 driven Cre specific in hematopoietic tissues. Some examples are listed in the following Table 1.
| Tissue/organ | cell | Promoter/enhancer | Reference |
| Cerebrum | Neuronal and glia cell precursors | Aldh1l1 | [12] |
| Cerebrum | CaMIIα | [13] | |
| GABAergic interneurons | Dlx1 | [14] | |
| GABAergic neurons | Dlx5/6 | [15-17] | |
| DRG | Gad2 | [18] | |
| Astrocyte | GFAP | [19, 20] | |
| CA3 pyramidal cells | Grik4 | [21] | |
| Arcuate nucleus | Lepr | [22] | |
| Neuronal and glia cell precursors | Nes | [23] | |
| DRG neurons | nNOS | [18] | |
| Retinal Müller glial cells | Pdgfrα | [24] | |
| Myelinating cells | PLP1 | [25] | |
| DRG neurons | Pv (Pvalb) | [18] | |
| GABAergic neurons | Slc17a6 | [26] | |
| GABAergic neurons | Sst, Vip | [17] | |
| Cerebellum | Cerebellar Purkinje cells | Pcp2 | [27] |
| Brain stem | Dopamine and serotonin neurons | Slc6a3 (DAT) | [28] |
| Serotonin neurons | ePet (Fev) | [29] | |
| Vagal sensory neurons | Npy2r | [30] | |
| Spinal cord | Mechanosensory dorsal horn | Cdh3, Htr6 | [31] |
| Skin | Basal layer of the epidermis | Krt5 | [32] |
| Epidermis keratinocytes | Krt10 | [33] | |
| Epidermal progenitors | Krt18 | [34] | |
| Immune system | Macrophage | Lyz2 | [35] |
| Dendritic cells | CD11c (Itgax) | [36] | |
| Mast cells | CMA1 | [37] | |
| T cells | CD2 | [38] | |
| CD4 | [39] | ||
| CD8a | [40] | ||
| Foxp3 | [41] | ||
| Lck | [42] | ||
| OX40 | [43] | ||
| B cells | CD19 | [44, 45] | |
| Hematopoietic cells | CD15 | [46] | |
| Musculoskeletal | Osteochondro progenitors | Twist2 (Dermol) | [47, 48] |
| Prrx1 | [49] | ||
| Osteoblasts | BGLOP | [50] | |
| Osteocytes | Dmp1 | [51] | |
| Osteoclasts | Ctsk | [52] | |
| Cardiomyocytes | NKX2.5 | [53] | |
| Muscle | ACTA1 (HSA) | [54] | |
| Digestive system | Stomach | ATP4b | [55] |
| Intestine | Car1 | [56] | |
| Intestine | Vil1 | [57] | |
| Liver | Alb | [58, 59] | |
| PanCreas | Ghrl | [60] | |
| Ins1 (MIP) | [61] | ||
| Ins2 (RIP) | [62] | ||
| Ptf1a | [63] | ||
| Urogenital system | Bladder | Upk2 | [64] |
| Kidney | Aqp2 | [65] | |
| Gdnf | [66] | ||
| Ggt1 | [67] | ||
| Ovary | Gdf9 | [68] | |
| Zp3 | [69] | ||
| Testis | Amh | [70] | |
| Hspa2 | [71] |
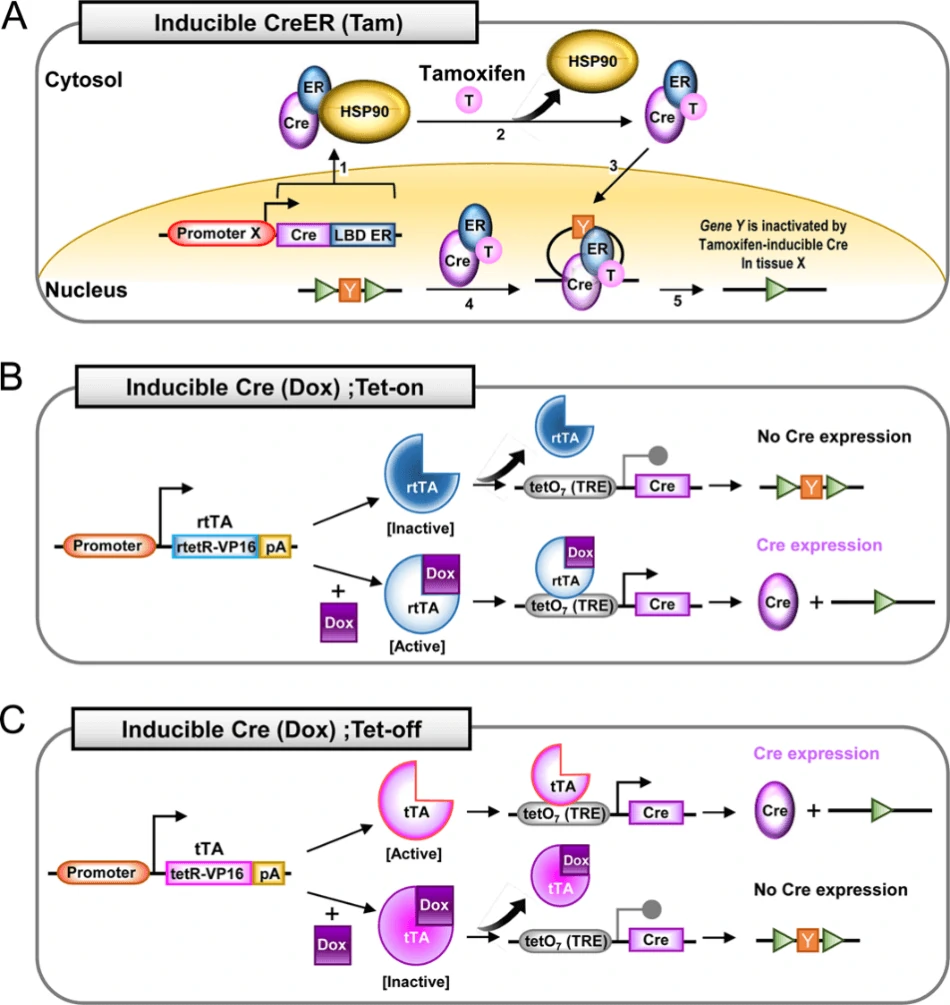
One inducible system is tamoxifen-inducible Cre-loxP system (Fig. 4A). When ubiquitous or tissue-specific promoter driven Cre is fused with the estrogen receptor containing a mutated ligand binding domain (ER-LBD), which is normally bound by heat shock protein 90 (HSP90), Cre-ER-HSP90 complex would be prevented from entering the nucleus. Once bound by the hormone (such as estrogen) or synthetic analogs (such as tamoxifen, T, or 4-hydoxytamoxifen, 4-OHT), ER will be released from HSP90. This enables the nuclear translocation of Cre-ERT complex and the following interaction between Cre and loxP sites [8, 32].
Another inducible system is tetracycline inducible Cre-loxP system, also known as doxycycline (dox; a tetracycline derivative)-inducible Cre system. This system has two mode: tet-on (Fig. 4B) and tet-off (Fig. 4C), responsible for the activation and inactivation of Cre gene, respectively [72, 73]. There are three elements orchestrating to control Cre expression in this system: reverse tetracycline-controlled transactivator (rtTA), tetracycline-controlled transactivator (tTA) and tetracycline responsive element (TRE), also named as a tetracycline operon (TetO). In tet-on system, ubiquitous or tissue-specific promoter driven rtTA binds to dox to turn on the transcription of Cre gene (Fig. 4B). On the contrary, in tet-off system, tTA binds to dox to turn off the transcription of Cre gene (Fig. 4C).
So far, a huge number of Cre transgenic lines have been established to combine with GOI loxP lines to generate conditional knockout mice or cell lines at the desired period and tissues in vitro or in vivo. Given that it is time and labor consuming to use Cre transgenic mice lines, viral vectors are convenient and versatile tools for the expression of Cre in certain cells or tissues, such as adeno-associated viral (AAV) vectors, as well as cell lines, such as adenovirus (Ad).
Summary
The Cre-loxP system, especially inducible tissue-specific knockout and viral vector-mediated Cre expression, has been highly utilized in genetics and cell biology research. Nevertheless, it seems that Cre-loxP system will continue to be prevalent in current and future research studies. GeneMedi is proficient in viral vector development and offers kinds of viral vector-based Cre tools, we can provide the best services and products if required.References
1.
Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981;150:467-486.
2.
Santoro SW, Schultz PG. Directed evolution of the site specificity of Cre recombinase. Proc Natl Acad Sci U S A. 2002;99:4185-4190.
3.
McLellan MA, Rosenthal NA, Pinto AR. Cre-loxP-Mediated Recombination: General Principles and Experimental Considerations. Curr Protoc Mouse Biol. 2017;7:1-12.
4.
Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99-109.
5.
Hall B, Limaye A, Kulkarni AB. Overview: generation of gene knockout mice. Curr Protoc Cell Biol. 2009;Chapter 19:Unit 19 12 19 12 11-17.
6.
Kos CH. Cre/loxP system for generating tissue-specific knockout mouse models. Nutr Rev. 2004;62:243-246.
7.
Kim H, Kim M, Im SK, Fang S. Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Lab Anim Res. 2018;34:147-159.
8.
Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71-80.
9.
Brocard J, Warot X, Wendling O, Messaddeq N, Vonesch JL, Chambon P, et al. Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc Natl Acad Sci U S A. 1997;94:14559-14563.
10.
Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427-1429.
11.
Soto M, Cai W, Konishi M, Kahn CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci U S A. 2019;116:6379-6384.
12.
Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817-9823.
13.
Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26:133-135.
14.
Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 2009;40:167-186.
15.
Zerucha T, Stuhmer T, Hatch G, Park BK, Long Q, Yu G, et al. A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J Neurosci. 2000;20:709-7
16.
Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455-466.
17.
Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995-1013.
18.
Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159.
19.
Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233-1241.
20.
Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030-1037.
21.
Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211-218.
22.
DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608-2613.
23.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99-103.
24.
Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, et al. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509:225-238.
25.
Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63-72.
26.
Vong L, Ye C, Yang Z, Choi B, Chua S, Jr., Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142-154.
27.
Zhang XM, Ng AH, Tanner JA, Wu WT, Copeland NG, Jenkins NA, et al. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis. 2004;40:45-51.
28.
Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27-32.
29.
Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472-16477.
30.
Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell. 2015;161:622-633.
31.
Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, et al. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell. 2017;168:295-310 e219.
32.
Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER
33.
Calleja C, Messaddeq N, Chapellier B, Yang H, Krezel W, Li M, et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006;20:1525-1538.
34.
Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91-100.
35.
Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265-277.
36.
Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653-1664.
37.
Musch W, Wege AK, Mannel DN, Hehlgans T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis. 2008;46:163-166.
38.
Vacchio MS, Wang L, Bouladoux N, Carpenter AC, Xiong Y, Williams LC, et al. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nat Immunol. 2014;15:947-956.
39.
Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917-929.
40.
Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9:1140-1147.
41.
Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667-1671.
42.
Hennet T, Hagen FK, Tabak LA, Marth JD. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci U S A. 1995;92:12070-12074.
43.
Klinger M, Kim JK, Chmura SA, Barczak A, Erle DJ, Killeen N. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. 2009;182:4581-4589.
44.
Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317-1318.
45.
Yasuda T, Wirtz T, Zhang B, Wunderlich T, Schmidt-Supprian M, Sommermann T, et al. Studying Epstein-Barr virus pathologies and immune surveillance by reconstructing EBV infection in mice. Cold Spring Harb Symp Quant Biol. 2013;78:259-263.
46.
Yang J, Hills D, Taylor E, Pfeffer K, Ure J, Medvinsky A. Transgenic tools for analysis of the haematopoietic system: knock-in CD45 reporter and deletor mice. J Immunol Methods. 2008;337:81-87.
47.
Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169-180.
48.
Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063-3074.
49.
Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77-80.
50.
Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Che
51.
Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320-325.
52.
Kim JE, Nakashima K, de Crombrugghe B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am J Pathol. 2004;165:1875-1882.
53.
Diehl F, Brown MA, van Amerongen MJ, Novoyatleva T, Wietelmann A, Harriss J, et al. Cardiac deletion of Smyd2 is dispensable for mouse heart development. PLoS One. 2010;5:e9748.
54.
Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, Melki J. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 1999;27:e27.
55.
Syder AJ, Karam SM, Mills JC, Ippolito JE, Ansari HR, Farook V, et al. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc Natl Acad
56.
Xue Y, Johnson R, Desmet M, Snyder PW, Fleet JC. Generation of a transgenic mouse for colorectal cancer research with intestinal cre expression limited to the large intestine. Mol Cancer Res. 2010;8:1095-1104.
57.
Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 200
58.
Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324-7329.
59.
Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci U S A. 2008;105:19378-19383.
60.
Arnes L, Hill JT, Gross S, Magnuson MA, Sussel L. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS One. 2012;7:e52026.
61.
Thorens B, Tarussio D, Maestro MA, Rovira M, Heikkila E, Ferrer J. Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia. 2015;58:558-565.
62.
Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:3
63.
Kopinke D, Brailsford M, Pan FC, Magnuson MA, Wright CV, Murtaugh LC. Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev Biol. 2012;362:57-64.
64.
Ayala de la Pena F, Kanasaki K, Kanasaki M, Tangirala N, Maeda G, Kalluri R. Loss of p53 and acquisition of angiogenic microRNA profile are insufficient to facilitate progression of bladder urothelial carcinoma in situ to invasive carcinoma
65.
Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, et al. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol. 2005;288:F912-920.
66.
Cebrian C, Asai N, D'Agati V, Costantini F. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep. 2014;7:127-137.
67.
Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341-350.
68.
Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469-1474.
69.
Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148-151.
70.
Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459-467.
71.
Inselman AL, Nakamura N, Brown PR, Willis WD, Goulding EH, Eddy EM. Heat shock protein 2 promoter drives Cre expression in spermatocytes of transgenic mice. Genesis. 2010;48:114-120.
72.
Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933-10938.
73.
Pluta K, Luce MJ, Bao L, Agha-Mohammadi S, Reiser J. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J Gene Med. 2005;7:803-817.
74.
Alisky JM, Hughes SM, Sauter SL, Jolly D, Dubensky TW, Jr., Staber PD, et al. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport. 2000;11:2669-2673.
75.
Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to differ
76.
Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proceedings of the
77.
Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. Journal of virology. 2004;78:6381-6388.
78.
Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1185
79.
Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Molecular therapy : the journal of the American Society of Gene Therapy. 2
80.
Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Molecular therapy : the journal of the American Society of G
81.
Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. Journal of virology. 2001;75:6615-6624.
82.
Rabinowitz JE, Bowles DE, Faust SM, Ledford JG, Cunningham SE, Samulski RJ. Cross-dressing the virion: the transcapsidation of adeno-associated virus serotypes functionally defines subgroups. Journal of virology. 2004;78:4421-4432.
83.
Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab. 2018;9:141-155.
84.
Chen L, Huang J, Zhao P, Persson AK, Dib-Hajj FB, Cheng X, et al. Conditional knockout of NaV1.6 in adult mice ameliorates neuropathic pain. Sci Rep. 2018;8:3845.
85.
Zhang Z, Ding X, Zhou Z, Qiu Z, Shi N, Zhou S, et al. Sirtuin 1 alleviates diabetic neuropathic pain by regulating synaptic plasticity of spinal dorsal horn neurons. Pain. 2019;160:1082-1092.
86.
Gallent EA, Steward O. Neuronal PTEN deletion in adult cortical neurons triggers progressive growth of cell bodies, dendrites, and axons. Exp Neurol. 2018;303:12-28.
87.
Lei Y, Wang J, Wang D, Li C, Liu B, Fang X, et al. SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression-related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefron
88.
Joubert R, Vignaud A, Le M, Moal C, Messaddeq N, Buj-Bello A. Site-specific Mtm1 mutagenesis by an AAV-Cre vector reveals that myotubularin is essential in adult muscle. Hum Mol Genet. 2013;22:1856-1866.
89.
Darnieder LM, Melon LC, Do T, Walton NL, Miczek KA, Maguire JL. Female-specific decreases in alcohol binge-like drinking resulting from GABAA receptor delta-subunit knockdown in the VTA. Sci Rep. 2019;9:8102.
90.
Yang P, Qin Y, Zhang W, Bian Z, Wang R. Sensorimotor Cortex Injection of Adeno-Associated Viral Vector Mediates Knockout of PTEN in Neurons of the Brain and Spinal Cord of Mice. J Mol Neurosci. 2015;57:470-476.
91.
Shen M, Jiang C, Liu P, Wang F, Ma L. Mesolimbic leptin signaling negatively regulates cocaine-conditioned reward. Transl Psychiatry. 2016;6:e972.
92.
Gonzalez-Reyes LE, Chiang CC, Zhang M, Johnson J, Arrillaga-Tamez M, Couturier NH, et al. Sonic Hedgehog is expressed by hilar mossy cells and regulates cellular survival and neurogenesis in the adult hippocampus. Sci Rep. 2019;9:17402.
93.
Khan MW, Priyadarshini M, Cordoba-Chacon J, Becker TC, Layden BT. Hepatic hexokinase domain containing 1 (HKDC1) improves whole body glucose tolerance and insulin sensitivity in pregnant mice. Biochim Biophys Acta Mol Basis Dis. 2019;1865:6
94.
Cao H, Chen X, Yang Y, Storm DR. Disruption of type 3 adenylyl cyclase expression in the hypothalamus leads to obesity. Integr Obes Diabetes. 2016;2:225-228.
95.
Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther. 2015;15:337-351.
96.
Sinnayah P, Lindley TE, Staber PD, Davidson BL, Cassell MD, Davisson RL. Targeted viral delivery of Cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain. Physiol Genomics. 2004;18:25-32.
97.
Xiang D, Tao L, Li Z. Modeling Breast Cancer via an Intraductal Injection of Cre-expressing Adenovirus into the Mouse Mammary Gland. J Vis Exp. 2019.
98.
Tao L, van Bragt MP, Laudadio E, Li Z. Lineage tracing of mammary epithelial cells using cell-type-specific cre-expressing adenoviruses. Stem Cell Reports. 2014;2:770-779.
99.
Penny GM, Cochran RB, Pihlajoki M, Kyronlahti A, Schrade A, Hakkinen M, et al. Probing GATA factor function in mouse Leydig cells via testicular injection of adenoviral vectors. Reproduction. 2017;154:455-467.
100.
Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14:1363-1369.
101.
Kobayashi N, Noguchi H, Westerman KA, Matsumura T, Watanabe T, Totsugawa T, et al. Efficient Cre/loxP site-specific recombination in a HepG2 human liver cell line. Cell Transplant. 2000;9:737-742.
102.
Gan Y, Zhang Y, Digirolamo DJ, Jiang J, Wang X, Cao X, et al. Deletion of IGF-I receptor (IGF-IR) in primary osteoblasts reduces GH-induced STAT5 signaling. Mol Endocrinol. 2010;24:644-656.
103.
Cao Y, Pan T, Chen X, Wu J, Guo N, Wang B. EP4 knockdown alleviates glomerulosclerosis through Smad and MAPK pathways in mesangial cells. Mol Med Rep. 2018;18:5141-5150.
104.
Gan Y, Paterson AJ, Zhang Y, Jiang J, Frank SJ. Functional collaboration of insulin-like growth factor-1 receptor (IGF-1R), but not insulin receptor (IR), with acute GH signaling in mouse calvarial cells. Endocrinology. 2014;155:1000-1009.
105.
Carofino BL, Justice MJ. Tissue-Specific Regulation of Oncogene Expression Using Cre-Inducible ROSA26 Knock-In Transgenic Mice. Curr Protoc Mouse Biol. 2015;5:187-204.
106.
Albert K, Voutilainen MH, Domanskyi A, Piepponen TP, Ahola S, Tuominen RK, et al. Downregulation of tyrosine hydroxylase phenotype after AAV injection above substantia nigra: Caution in experimental models of Parkinson's disease. J Neurosci Res. 2019;97:346-3
107.
Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981-1990.
108.
Dugue GP, Akemann W, Knopfel T. A comprehensive concept of optogenetics. Prog Brain Res. 2012;196:1-28.
109.
Kim B, Lin MZ. Optobiology: optical control of biological processes via protein engineering. Biochem Soc Trans. 2013;41:1183-1188.
110.
DePuy SD, Stornetta RL, Bochorishvili G, Deisseroth K, Witten I, Coates M, et al. Glutamatergic neurotransmission between the C1 neurons and the parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus. J Neurosci. 2013;33:1486-1497.




